Impaired learning resulting from respiratory syncytial virus infection
- PMID: 23650398
- PMCID: PMC3670318
- DOI: 10.1073/pnas.1217508110
Impaired learning resulting from respiratory syncytial virus infection
Abstract
Respiratory syncytial virus (RSV) is the major cause of respiratory illness in infants worldwide. Neurologic alterations, such as seizures and ataxia, have been associated with RSV infection. We demonstrate the presence of RSV proteins and RNA in zones of the brain--such as the hippocampus, ventromedial hypothalamic nucleus, and brainstem--of infected mice. One month after disease resolution, rodents showed behavioral and cognitive impairment in marble burying (MB) and Morris water maze (MWM) tests. Our data indicate that the learning impairment caused by RSV is a result of a deficient induction of long-term potentiation in the hippocampus of infected animals. In addition, immunization with recombinant bacillus Calmette-Guérin (BCG) expressing RSV nucleoprotein prevented behavioral disorders, corroborating the specific effect of RSV infection over the central nervous system. Our findings provide evidence that RSV can spread from the airways to the central nervous system and cause functional alterations to the brain, both of which can be prevented by proper immunization against RSV.
Keywords: LTD; LTP; behavior; cognition; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


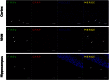



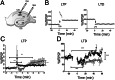
 ) rats. (D) LTD in the CA1 region of the hippocampus. Data in graph show the LTD elicited by low-frequency stimulation (5 Hz stimulation for 3 min given twice with a 3-min interval) for RSV-infected (■) or mock control (
) rats. (D) LTD in the CA1 region of the hippocampus. Data in graph show the LTD elicited by low-frequency stimulation (5 Hz stimulation for 3 min given twice with a 3-min interval) for RSV-infected (■) or mock control ( ) rats. (Scale bar, 0.5 mV, 10 ms.) Statistical analysis, unpaired t test; ***P < 0.0001, n = 7 slides, 4 rats for each group.
) rats. (Scale bar, 0.5 mV, 10 ms.) Statistical analysis, unpaired t test; ***P < 0.0001, n = 7 slides, 4 rats for each group.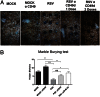

References
-
- Bueno SM, et al. Host immunity during RSV pathogenesis. Int Immunopharmacol. 2008;8(10):1320–1329. - PubMed
-
- González PA, Bueno SM, Riedel CA, Kalergis AM. Impairment of T cell immunity by the respiratory syncytial virus: Targeting virulence mechanisms for therapy and prophylaxis. Curr Med Chem. 2009;16(34):4609–4625. - PubMed
-
- Sweetman LL, Ng YT, Butler IJ, Bodensteiner JB. Neurologic complications associated with respiratory syncytial virus. Pediatr Neurol. 2005;32(5):307–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

