Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor
- PMID: 23650416
- PMCID: PMC4881332
- DOI: 10.1200/JCO.2012.43.7251
Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor
Abstract
Purpose: Entinostat is an oral isoform selective histone deacetylase inhibitor that targets resistance to hormonal therapies in estrogen receptor-positive (ER+) breast cancer. This randomized, placebo-controlled, phase II study evaluated entinostat combined with the aromatase inhibitor exemestane versus exemestane alone.
Patients and methods: Postmenopausal women with ER+ advanced breast cancer progressing on a nonsteroidal aromatase inhibitor were randomly assigned to exemestane 25 mg daily plus entinostat 5 mg once per week (EE) or exemestane plus placebo (EP). The primary end point was progression-free survival (PFS). Blood was collected in a subset of patients for evaluation of protein lysine acetylation as a biomarker of entinostat activity.
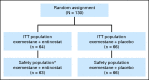
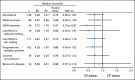
Results: One hundred thirty patients were randomly assigned (EE group, n = 64; EP group, n = 66). Based on intent-to-treat analysis, treatment with EE improved median PFS to 4.3 months versus 2.3 months with EP (hazard ratio [HR], 0.73; 95% CI, 0.50 to 1.07; one-sided P = .055; two-sided P = .11 [predefined significance level of .10, one-sided]). Median overall survival was an exploratory end point and improved to 28.1 months with EE versus 19.8 months with EP (HR, 0.59; 95% CI, 0.36 to 0.97; P = .036). Fatigue and neutropenia were the most frequent grade 3/4 toxicities. Treatment discontinuation because of adverse events was higher in the EE group versus the EP group (11% v 2%). Protein lysine hyperacetylation in the EE biomarker subset was associated with prolonged PFS.
Conclusion: Entinostat added to exemestane is generally well tolerated and demonstrated activity in patients with ER+ advanced breast cancer in this signal-finding phase II study. Acetylation changes may provide an opportunity to maximize clinical benefit with entinostat. Plans for a confirmatory study are underway.
Trial registration: ClinicalTrials.gov NCT00676663.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor–positive, advanced breast cancer: Results from EFECT. J Clin Oncol. 2008;26:1664–1670. - PubMed
-
- Chung EJ, Lee S, Sausville EA, et al. Histone deacetylase inhibitor pharmacodynamic analysis by multiparameter flow cytometry. Ann Clin Lab Sci. 2005;35:397–406. - PubMed
-
- Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

