Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in an intracranial model of breast cancer
- PMID: 23650496
- PMCID: PMC3641071
- DOI: 10.1371/journal.pone.0061359
Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in an intracranial model of breast cancer
Abstract
Introduction: Breast cancer brain metastases (BCBM) are a challenging consequence of advanced BC. Nanoparticle agents, including liposomes, have shown enhanced delivery to solid tumors and brain. We compared pharmacokinetics (PK) and efficacy of PEGylated liposomal doxorubicin (PLD) with non-liposomal doxorubicin (NonL-doxo) in an intracranial model of BC.
Methods: Athymic mice were inoculated intracerebrally with MDA-MB-231-BR-luciferase-expressing cells. Tumor-bearing mice were administered PLD or NonL-doxo at 6 mg/kg IV × 1 and were euthanized prior to and 0.083, 1, 3, 6, 24, 72 and 96 h post-treatment. Samples were processed to measure sum total doxorubicin via HPLC. PLD and NonL-doxo were administered IV weekly as single agents (6 mg/kg) or in combination (4.5 mg/kg) with the PARP inhibitor, ABT-888, PO 25 mg/kg/day. Efficacy was assessed by survival and bioluminescence.
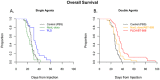
Results: Treatment with PLD resulted in approximately 1,500-fold higher plasma and 20-fold higher intracranial tumor sum total doxorubicin AUC compared with NonL-doxo. PLD was detected at 96 h; NonL-doxo was undetectable after 24 h in plasma and tumor. Median survival of PLD-treated animals was 32 days (d, [CI] 31-38), which was significantly longer than controls (26d [CI 25-28]; p = 0.0012) or NonL-doxo treatment (23.5d [CI 18-28], p = 0.0002). Combination treatment with PLD/ABT-888 yielded improved survival compared to NonL-doxo/ABT-888 (35d [CI 31-38] versus 29.5d [CI 25-34]; p = 0.006).
Conclusions: PLD provides both PK and efficacy advantage over NonL-doxo in the treatment of an in vivo model of BCBM. The results provide preclinical rationale to translate findings into early phase trials of PLD, with or without ABT-888, for patients with BCBM.
Conflict of interest statement
Figures


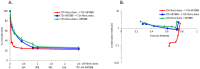

References
-
- Lin NU, Bellon JR, Winer EP (2004) CNS metastases in breast cancer. Journal of clinical oncology 22: 3608–3617. - PubMed
-
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, et al. (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97: 2972–2977. - PubMed
-
- Niwińska A, Murawska M, Pogoda K (2010) Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Annals of Oncology 21: 942–948. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

