A randomized, observer-blinded immunogenicity trial of Cervarix(®) and Gardasil(®) Human Papillomavirus vaccines in 12-15 year old girls
- PMID: 23650505
- PMCID: PMC3641072
- DOI: 10.1371/journal.pone.0061825
A randomized, observer-blinded immunogenicity trial of Cervarix(®) and Gardasil(®) Human Papillomavirus vaccines in 12-15 year old girls
Abstract
Background: The current generation of Human Papillomavirus (HPV) vaccines, Cervarix® and Gardasil®, exhibit a high degree of efficacy in clinical trials against the two high-risk (HR) genotypes represented in the vaccines (HPV16 and HPV18). High levels of neutralizing antibodies are elicited against the vaccine types, consistent with preclinical data showing that neutralizing antibodies can mediate type-specific protection in the absence of other immune effectors. The vaccines also confer protection against some closely related non-vaccine HR HPV types, although the vaccines appear to differ in their degree of cross-protection. The mechanism of vaccine-induced cross-protection is unknown. This study sought to compare the breadth and magnitudes of neutralizing antibodies against non-vaccine types elicited by both vaccines and establish whether such antibodies could be detected in the genital secretions of vaccinated individuals.
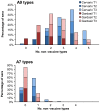
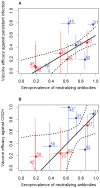
Methods and findings: Serum and genital samples were collected from 12-15 year old girls following vaccination with either Cervarix® (n = 96) or Gardasil® (n = 102) HPV vaccine. Serum-neutralizing antibody responses against non-vaccine HPV types were broader and of higher magnitude in the Cervarix®, compared to the Gardasil®, vaccinated individuals. Levels of neutralizing and binding antibodies in genital secretions were closely associated with those found in the serum (r = 0.869), with Cervarix® having a median 2.5 (inter-quartile range, 1.7-3.5) fold higher geometric mean HPV-specific IgG ratio in serum and genital samples than Gardasil® (p = 0.0047). There was a strong positive association between cross-neutralizing antibody seropositivity and available HPV vaccine trial efficacy data against non-vaccine types.
Conclusions: These data demonstrate for the first time that cross-neutralizing antibodies can be detected at the genital site of infection and support the possibility that cross-neutralizing antibodies play a role in the cross-protection against HPV infection and disease that has been reported for the current HPV vaccines.
Trial registration: ClinicalTrials.gov NCT00956553.
Conflict of interest statement
Figures


References
-
- Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, et al. (2011) Worldwide burden of cervical cancer in 2008. Ann Oncol 22: 2675–2686. - PubMed
-
- Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. - PubMed
-
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM (2010) Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 128: 927–935. - PubMed
-
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, et al. (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11: 1048–1056. - PubMed
-
- Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, et al... (2012) Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int J Cancer. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

