Sleep: a synchrony of cell activity-driven small network states
- PMID: 23651209
- PMCID: PMC3713192
- DOI: 10.1111/ejn.12238
Sleep: a synchrony of cell activity-driven small network states
Abstract
We posit a bottom-up sleep-regulatory paradigm in which state changes are initiated within small networks as a consequence of local cell activity. Bottom-up regulatory mechanisms are prevalent throughout nature, occurring in vastly different systems and levels of organization. Synchronization of state without top-down regulation is a fundamental property of large collections of small semi-autonomous entities. We posit that such synchronization mechanisms are sufficient and necessary for whole-organism sleep onset. Within the brain we posit that small networks of highly interconnected neurons and glia, for example cortical columns, are semi-autonomous units oscillating between sleep-like and wake-like states. We review evidence showing that cells, small networks and regional areas of the brain share sleep-like properties with whole-animal sleep. A testable hypothesis focused on how sleep is initiated within local networks is presented. We posit that the release of cell activity-dependent molecules, such as ATP and nitric oxide, into the extracellular space initiates state changes within the local networks where they are produced. We review mechanisms of ATP induction of sleep-regulatory substances and their actions on receptor trafficking. Finally, we provide an example of how such local metabolic and state changes provide mechanistic explanations for clinical conditions, such as insomnia.
Keywords: ATP; brain imaging; cerebral blood flow; cytokine; receptor.
© 2013 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures

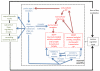

References
-
- Aalto J, Kiianmaa K. REM-sleep deprivation-induced increase in ethanol intake: role of brain monoaminergic neurons. Alcohol. 1986;3:377–381. - PubMed
-
- Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, Szymusiak R. Interleukin-1 beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: Role in sleep regulation. Eur. J. Neurosci. 2004;20:207–216. - PubMed
-
- Balkin TJ, Braun AR, Wesensten NJ, Jeffries K, Varga M, Baldwin P, et al. The process of awakening: a PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain. 2002;125:2308–2319. - PubMed
-
- Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet. 2000;356:484–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD036520/HD/NICHD NIH HHS/United States
- R01 HD036520/HD/NICHD NIH HHS/United States
- AG020677/AG/NIA NIH HHS/United States
- DA029565/DA/NIDA NIH HHS/United States
- P01 AG020677/AG/NIA NIH HHS/United States
- K99 DA029565/DA/NIDA NIH HHS/United States
- R00 DA029565/DA/NIDA NIH HHS/United States
- RR024153/RR/NCRR NIH HHS/United States
- MH024657/MH/NIMH NIH HHS/United States
- MH60263/MH/NIMH NIH HHS/United States
- NS025378/NS/NINDS NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- R01 NS025378/NS/NINDS NIH HHS/United States
- R01 MH060263/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

