Discovery of N-{4-[(3-hydroxyphenyl)-3-methylpiperazin-1-yl]methyl-2-methylpropyl}-4-phenoxybenzamide analogues as selective kappa opioid receptor antagonists
- PMID: 23651437
- PMCID: PMC3701944
- DOI: 10.1021/jm400275h
Discovery of N-{4-[(3-hydroxyphenyl)-3-methylpiperazin-1-yl]methyl-2-methylpropyl}-4-phenoxybenzamide analogues as selective kappa opioid receptor antagonists
Abstract
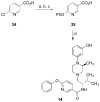
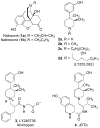
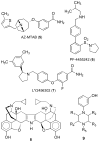
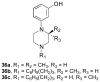
There is continuing interest in the discovery and development of new κ opioid receptor antagonists. We recently reported that N-substituted 3-methyl-4-(3-hydroxyphenyl)piperazines were a new class of opioid receptor antagonists. In this study, we report the syntheses of two piperazine JDTic-like analogues. Evaluation of the two compounds in an in vitro [(35)S]GTPγS binding assay showed that neither compound showed the high potency and κ opioid receptor selectivity of JDTic. A library of compounds using the core scaffold 21 was synthesized and tested for their ability to inhibit [(35)S]GTPγS binding stimulated by the selective κ opioid agonist U69,593. These studies led to N-[(1S)-1-{[(3S)-4-(3-hydroxyphenyl)-3-methylpiperazin-1-yl]methyl}-2-methylpropyl]-4-phenoxybenzamide (11a), a compound that showed good κ opioid receptor antagonist properties. An SAR study based on 11a provided 28 novel analogues. Evaluation of these 28 compounds in the [(35)S]GTPγS binding assay showed that several of the analogues were potent and selective κ opioid receptor antagonists.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Discovery of the first small-molecule opioid pan antagonist with nanomolar affinity at mu, delta, kappa, and nociceptin opioid receptors.ACS Chem Neurosci. 2015 Apr 15;6(4):646-57. doi: 10.1021/cn500367b. Epub 2015 Feb 18. ACS Chem Neurosci. 2015. PMID: 25635572 Free PMC article.
-
Identification of (3R)-7-hydroxy-N-((1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)- 3,4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl)-1,2,3,4-tetrahydro- 3-isoquinolinecarboxamide as a novel potent and selective opioid kappa receptor antagonist.J Med Chem. 2003 Jul 3;46(14):3127-37. doi: 10.1021/jm030094y. J Med Chem. 2003. PMID: 12825951
-
Analogues of (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic). Synthesis and in vitro and in vivo opioid receptor antagonist activity.J Med Chem. 2010 Jul 22;53(14):5290-301. doi: 10.1021/jm1004978. J Med Chem. 2010. PMID: 20568781 Free PMC article.
-
The discovery and development of the N-substituted trans-3,4-dimethyl-4-(3'-hydroxyphenyl)piperidine class of pure opioid receptor antagonists.ChemMedChem. 2014 Aug;9(8):1638-54. doi: 10.1002/cmdc.201402142. Epub 2014 Jun 30. ChemMedChem. 2014. PMID: 24981721 Free PMC article. Review.
-
Antagonist for the Kappa Opioid Receptor.2010 Oct 31 [updated 2011 May 26]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2010 Oct 31 [updated 2011 May 26]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 22091479 Free Books & Documents. Review.
Cited by
-
Structural Characterization of KOR Inactive and Active States for 3D Pharmacology and Drug Discovery.Handb Exp Pharmacol. 2022;271:41-64. doi: 10.1007/164_2021_461. Handb Exp Pharmacol. 2022. PMID: 33945028 Review.
-
Searching for Synthetic Opioid Rescue Agents: Identification of a Potent Opioid Agonist with Reduced Respiratory Depression.J Med Chem. 2024 Jun 13;67(11):9173-9193. doi: 10.1021/acs.jmedchem.4c00333. Epub 2024 May 29. J Med Chem. 2024. PMID: 38810170 Free PMC article.
-
Design, synthesis, and biological evaluation of (3R)-1,2,3,4-tetrahydro-7-hydroxy-N-[(1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl]-3-isoquinolinecarboxamide (JDTic) analogues: in vitro pharmacology and ADME profile.J Med Chem. 2014 Sep 11;57(17):7367-81. doi: 10.1021/jm5008177. Epub 2014 Aug 25. J Med Chem. 2014. PMID: 25133923 Free PMC article.
-
N-(3-Hydroxyphenyl)-3,8-diazabicyclooctanes as opioid receptors probes. 1. Investigation of the phenolic hydroxyl group.Eur J Med Chem. 2025 Nov 15;298:117991. doi: 10.1016/j.ejmech.2025.117991. Epub 2025 Jul 23. Eur J Med Chem. 2025. PMID: 40730064
-
Recent Advances in the Synthesis and Applications of m-Aryloxy Phenols.Molecules. 2023 Mar 15;28(6):2657. doi: 10.3390/molecules28062657. Molecules. 2023. PMID: 36985628 Free PMC article. Review.
References
-
- Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–592. - PubMed
-
- Aldrich JV, Vigil-Cruz SC. Narcotic Analgesics. In: Abraham DJ, editor. Burger’s Medicinal Chemistry and Drug Discovery. 6. Vol. 6. John Wiley & Sons; New York, NY: 2003. pp. 329–481. Chapter 7.
-
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. - PubMed
-
- Zimmerman DM, Nickander R, Horng JS, Wong DT. New structural concepts for narcotic antagonists defined in a 4-phenylpiperidine series. Nature. 1978;275:332–334. - PubMed
-
- Thomas JB, Mascarella SW, Rothman RB, Partilla JS, Xu H, McCullough KB, Dersch CM, Cantrell BE, Zimmerman DM, Carroll FI. Investigation of the N-substituent conformation governing potency and mu receptor subtype-selectivity in (+)-(3R, 4R)-dimethyl-4-(3-hydroxyphenyl)piperidine opioid antagonists. J Med Chem. 1998;41:1980–1990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

