A survivin-associated adaptive response in radiation therapy
- PMID: 23651635
- PMCID: PMC3721325
- DOI: 10.1158/0008-5472.CAN-12-4640
A survivin-associated adaptive response in radiation therapy
Abstract
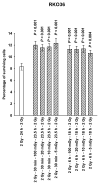
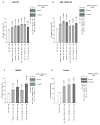
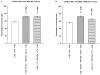
Adaptive responses can be induced in cells by very low doses of ionizing radiation resulting in an enhanced resistance to much larger exposures. The inhibitor of apoptosis protein, survivin, has been implicated in many adaptive responses to cellular stress. Computerized axial tomography used in image-guided radiotherapy to position and monitor tumor response uses very low radiation doses ranging from 0.5 to 100 mGy. We investigated the ability of these very low radiation doses administered along with two 2 Gy doses separated by 24 hours, a standard conventional radiotherapy dosing schedule, to initiate adaptive responses resulting in the elevation of radiation resistance in exposed cells. Human colon carcinoma (RKO36), mouse sarcoma (SA-NH), along with transformed mouse embryo fibroblasts, wild type or cells lacking functional tumor necrosis factor receptors 1 and 2 were used to assess their relative ability to express an adaptive response when grown either to confluence in vitro or as tumors in the flank of C57BL/6 mice. The survival of each of these cells was elevated from 5% to 20% (P ≤ 0.05) as compared to cells not receiving a 100 mGy or lesser dose. In addition, the cells exposed to 100 mGy exhibited elevations in survivin levels, reductions in apoptosis frequencies, and loss of an adaptive response if transfected with survivin siRNA. This survivin-mediated adaptive response has the potential for affecting outcomes if regularly induced throughout a course of image guided radiation therapy.
©2013 AACR.
Conflict of interest statement
Conflict of Interest: Dr. David J. Grdina is a paid consultant to Pinnacle Biologics and Drs. Grdina and Murley are minority equity partners in Pinnacle Oncology LLC regarding the potential novel uses of amifostine. Dr. Weichselbaum is a consultant to Reflexion, a radiotherapy company.
Dr. David J. Grdina is a paid consultant to Pinnacle Biologics and Drs. Grdina and Murley are minority equity partners in Pinnacle Oncology LLC regarding the potential novel uses of amifostine. Dr. Weichselbaum is a consultant to Reflexion, a radiotherapy company.
Figures


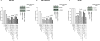
Similar articles
-
Very low doses of ionizing radiation and redox associated modifiers affect survivin-associated changes in radiation sensitivity.Free Radic Biol Med. 2016 Oct;99:110-119. doi: 10.1016/j.freeradbiomed.2016.07.009. Epub 2016 Jul 15. Free Radic Biol Med. 2016. PMID: 27427516 Free PMC article.
-
NFκB and Survivin-Mediated Radio-Adaptive Response.Radiat Res. 2015 Apr;183(4):391-7. doi: 10.1667/RR14002.1. Epub 2015 Mar 12. Radiat Res. 2015. PMID: 25763931
-
A manganese superoxide dismutase (SOD2)-mediated adaptive response.Radiat Res. 2013 Feb;179(2):115-24. doi: 10.1667/RR3126.2. Epub 2012 Dec 13. Radiat Res. 2013. PMID: 23237540 Free PMC article.
-
ROS modifiers and NOX4 affect the expression of the survivin-associated radio-adaptive response.Free Radic Biol Med. 2018 Aug 1;123:39-52. doi: 10.1016/j.freeradbiomed.2018.04.547. Epub 2018 Apr 13. Free Radic Biol Med. 2018. PMID: 29660403
-
Insights on the Radiation-Induced Adaptive Response at the Cellular Level and Its Implications in Cancer Therapy.Cytogenet Genome Res. 2023;163(5-6):257-273. doi: 10.1159/000534500. Epub 2023 Oct 31. Cytogenet Genome Res. 2023. PMID: 37906989 Review.
Cited by
-
Exploiting DNA repair pathways for tumor sensitization, mitigation of resistance, and normal tissue protection in radiotherapy.Cancer Drug Resist. 2021;4(2):244-263. doi: 10.20517/cdr.2020.89. Epub 2021 Jun 19. Cancer Drug Resist. 2021. PMID: 34337349 Free PMC article.
-
Adaptive Response: A Scoping Review of Its Implications in Medicine, Space Exploration, and Beyond.Dose Response. 2025 Jul 19;23(3):15593258251360051. doi: 10.1177/15593258251360051. eCollection 2025 Jul-Sep. Dose Response. 2025. PMID: 40686626 Free PMC article. Review.
-
Survivin Small Molecules Inhibitors: Recent Advances and Challenges.Molecules. 2023 Feb 1;28(3):1376. doi: 10.3390/molecules28031376. Molecules. 2023. PMID: 36771042 Free PMC article. Review.
-
Effects of ionizing radiation on biological molecules--mechanisms of damage and emerging methods of detection.Antioxid Redox Signal. 2014 Jul 10;21(2):260-92. doi: 10.1089/ars.2013.5489. Epub 2014 Feb 21. Antioxid Redox Signal. 2014. PMID: 24382094 Free PMC article. Review.
-
Very low doses of ionizing radiation and redox associated modifiers affect survivin-associated changes in radiation sensitivity.Free Radic Biol Med. 2016 Oct;99:110-119. doi: 10.1016/j.freeradbiomed.2016.07.009. Epub 2016 Jul 15. Free Radic Biol Med. 2016. PMID: 27427516 Free PMC article.
References
-
- Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double strand breaks in DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53(1):39–47. - PubMed
-
- Dixon RG, Ogden K. Optimizing dose in computed tomographic procedures. Tech Vasc Interv Radiol. 2010;13(3):172–5. - PubMed
-
- Phan N, De Lisio M, Parise G, Boreham DR. Biological effects and adaptive response from single and repeated computed tomography scans in reticulocytes and bone marrow of C57BL/6 mice. Radiat Res. 2012;177:164–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

