Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media
- PMID: 23651806
- PMCID: PMC3751429
- DOI: 10.1186/1472-6750-13-39
Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media
Abstract
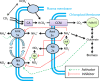
Background: Lack of accounting for proton uptake and secretion has confounded interpretation of the stoichiometry of photosynthetic growth of algae. This is also problematic for achieving growth of microalgae to high cell concentrations which is necessary to improve productivity and the economic feasibility of commercial-scale chemical production systems. Since microalgae are capable of consuming both nitrate and ammonium, this represents an opportunity to balance culture pH based on a nitrogen feeding strategy that does not utilize gas-phase CO₂ buffering. Stoichiometry suggests that approximately 36 weight%NH₄⁺ (balance nitrogen as NO₃⁻) would minimize the proton imbalance and permit high-density photoautotrophic growth as it does in higher plant tissue culture. However, algal media almost exclusively utilize nitrate, and ammonium is often viewed as 'toxic' to algae.
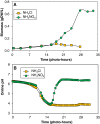
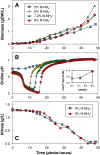
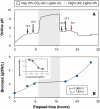
Results: The microalgae Chlorella vulgaris and Chlamydomonas reinhardtii exclusively utilize ammonium when both ammonium and nitrate are provided during growth on excess CO₂. The resulting proton imbalance from preferential ammonium utilization causes the pH to drop too low to sustain further growth when ammonium was only 9% of the total nitrogen (0.027 gN-NH₄⁺/L). However, providing smaller amounts of ammonium sequentially in the presence of nitrate maintained the pH of a Chlorella vulgaris culture for improved growth on 0.3 gN/L to 5 gDW/L under 5% CO₂ gas-phase supplementation. Bioreactor pH dynamics are shown to be predictable based on simple nitrogen assimilation as long as there is sufficient CO₂ availability.
Conclusions: This work provides both a media formulation and a feeding strategy with a focus on nitrogen metabolism and regulation to support high-density algal culture without buffering. The instability in culture pH that is observed in microalgal cultures in the absence of buffers can be overcome through alternating utilization of ammonium and nitrate. Despite the highly regulated array of nitrogen transporters, providing a nitrogen source with a balanced degree of reduction minimizes pH fluctuations. Understanding and accommodating the behavior of nitrogen utilization in microalgae is key to avoiding 'culture crash' and reliance on gas phase CO₂ buffering, which becomes both ineffective and cost-prohibitive for commercial-scale algal culture.
Figures




Similar articles
-
Comparative Shotgun Proteomic Analysis of Wastewater-Cultured Microalgae: Nitrogen Sensing and Carbon Fixation for Growth and Nutrient Removal in Chlamydomonas reinhardtii.J Proteome Res. 2015 Aug 7;14(8):3051-67. doi: 10.1021/pr501316h. Epub 2015 Jul 8. J Proteome Res. 2015. PMID: 25997359
-
Growth of Chlorella vulgaris and nutrient removal in the wastewater in response to intermittent carbon dioxide.Chemosphere. 2017 Nov;186:977-985. doi: 10.1016/j.chemosphere.2017.07.160. Epub 2017 Jul 31. Chemosphere. 2017. PMID: 28835006
-
Cultivation factors and population size control the uptake of nitrogen by the microalgae Chlorella vulgaris when interacting with the microalgae growth-promoting bacterium Azospirillum brasilense.FEMS Microbiol Ecol. 2005 Oct 1;54(2):197-203. doi: 10.1016/j.femsec.2005.03.014. FEMS Microbiol Ecol. 2005. PMID: 16332319
-
Isolation of indole-3-acetic acid-producing Azospirillum brasilense from Vietnamese wet rice: Co-immobilization of isolate and microalgae as a sustainable biorefinery.J Biotechnol. 2022 Apr 10;349:12-20. doi: 10.1016/j.jbiotec.2022.03.007. Epub 2022 Mar 21. J Biotechnol. 2022. PMID: 35331729 Review.
-
Utilizing CO2 in industrial off-gas for microalgae cultivation: considerations and solutions.Crit Rev Biotechnol. 2024 Aug;44(5):910-923. doi: 10.1080/07388551.2023.2233692. Epub 2023 Jul 27. Crit Rev Biotechnol. 2024. PMID: 37500178 Review.
Cited by
-
Fixing the Broken Phosphorus Cycle: Wastewater Remediation by Microalgal Polyphosphates.Front Plant Sci. 2020 Jun 30;11:982. doi: 10.3389/fpls.2020.00982. eCollection 2020. Front Plant Sci. 2020. PMID: 32695134 Free PMC article. Review.
-
Prevalent pH Controls the Capacity of Galdieria maxima to Use Ammonia and Nitrate as a Nitrogen Source.Plants (Basel). 2020 Feb 11;9(2):232. doi: 10.3390/plants9020232. Plants (Basel). 2020. PMID: 32054108 Free PMC article.
-
Algae drive convergent bacterial community assembly at low dilution frequency.iScience. 2023 May 16;26(6):106879. doi: 10.1016/j.isci.2023.106879. eCollection 2023 Jun 16. iScience. 2023. PMID: 37275519 Free PMC article.
-
Carbon and energy balance of biotechnological glycolate production from microalgae in a pre-industrial scale flat panel photobioreactor.Biotechnol Biofuels Bioprod. 2024 Mar 15;17(1):42. doi: 10.1186/s13068-024-02479-4. Biotechnol Biofuels Bioprod. 2024. PMID: 38486283 Free PMC article.
-
Development of a fermentation strategy to enhance the catalytic efficiency of recombinant Escherichia coli for l-2-aminobutyric acid production.3 Biotech. 2021 Aug;11(8):387. doi: 10.1007/s13205-021-02937-y. Epub 2021 Jul 28. 3 Biotech. 2021. PMID: 34350092 Free PMC article.
References
-
- Pulz O, Scheibenbogen K, Institut IGV, Scheunert-allee A, Rehbriicke B. Photobioreactors: design and performance with respect to light energy input. Bioprocess and Algae Reactor Technology. 1998;59:123–152. doi: 10.1007/BFb0102298. - DOI
-
- McLaughlin S, Bouton J, Bransby D, Conger B, Ocumpaugh W, Parrish D, Taliaferro C, Vogel K, Wullschleger S. Developing switchgrass as a bioenergy crop. Perspectives on new crops and new uses. ASHS Press; 1999. pp. 282–299.
-
- Varma A, Palsson B. Metabolic flux balancing: basic concepts, scientific and practical use. Bio/technology. 1994;12:994–998. doi: 10.1038/nbt1094-994. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

