Enhanced insulin clearance in mice lacking TRPM8 channels
- PMID: 23651844
- PMCID: PMC3725566
- DOI: 10.1152/ajpendo.00542.2012
Enhanced insulin clearance in mice lacking TRPM8 channels
Abstract
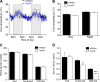
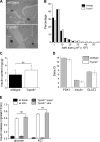
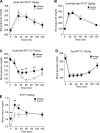
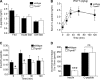
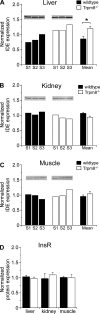
Blood glucose concentration is tightly regulated by the rate of insulin secretion and clearance, a process partially controlled by sensory neurons serving as metabolic sensors in relevant tissues. The activity of these neurons is regulated by the products of metabolism which regulate transmitter release, and recent evidence suggests that neuronally expressed ion channels of the transient receptor potential (TRP) family function in this critical process. Here, we report the novel finding that the cold and menthol-gated channel TRPM8 is necessary for proper insulin homeostasis. Mice lacking TRPM8 respond normally to a glucose challenge while exhibiting prolonged hypoglycemia in response to insulin. Additionally, Trpm8-/- mice have increased rates of insulin clearance compared with wild-type animals and increased expression of insulin-degrading enzyme in the liver. TRPM8 channels are not expressed in the liver, but TRPM8-expressing sensory afferents innervate the hepatic portal vein, suggesting a TRPM8-mediated neuronal control of liver insulin clearance. These results demonstrate that TRPM8 is a novel regulator of serum insulin and support the role of sensory innervation in metabolic homeostasis.
Keywords: TRPM8; clearance; insulin; neuronal; sensitivity.
Figures


Similar articles
-
Phospholipase C δ4 regulates cold sensitivity in mice.J Physiol. 2016 Jul 1;594(13):3609-28. doi: 10.1113/JP272321. Epub 2016 May 29. J Physiol. 2016. PMID: 27062607 Free PMC article.
-
TRPA1 expression levels and excitability brake by KV channels influence cold sensitivity of TRPA1-expressing neurons.Neuroscience. 2017 Jun 14;353:76-86. doi: 10.1016/j.neuroscience.2017.04.001. Epub 2017 Apr 10. Neuroscience. 2017. PMID: 28408328 Free PMC article.
-
A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia.J Neurosci. 2013 Feb 13;33(7):2837-48. doi: 10.1523/JNEUROSCI.1943-12.2013. J Neurosci. 2013. PMID: 23407943 Free PMC article.
-
Scraping through the ice: uncovering the role of TRPM8 in cold transduction.Am J Physiol Regul Integr Comp Physiol. 2011 Jun;300(6):R1278-87. doi: 10.1152/ajpregu.00631.2010. Epub 2011 Mar 16. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21411765 Free PMC article. Review.
-
TRPM8: from cold to cancer, peppermint to pain.Curr Pharm Biotechnol. 2011 Jan 1;12(1):68-77. doi: 10.2174/138920111793937961. Curr Pharm Biotechnol. 2011. PMID: 20932257 Review.
Cited by
-
Ecological Sensing Through Taste and Chemosensation Mediates Inflammation: A Biological Anthropological Approach.Adv Nutr. 2020 Nov 16;11(6):1671-1685. doi: 10.1093/advances/nmaa078. Adv Nutr. 2020. PMID: 32647890 Free PMC article. Review.
-
Progress in the Structural Basis of thermoTRP Channel Polymodal Gating.Int J Mol Sci. 2023 Jan 1;24(1):743. doi: 10.3390/ijms24010743. Int J Mol Sci. 2023. PMID: 36614186 Free PMC article. Review.
-
Lack of TRPV1 Channel Modulates Mouse Gene Expression and Liver Proteome with Glucose Metabolism Changes.Int J Mol Sci. 2022 Jun 24;23(13):7014. doi: 10.3390/ijms23137014. Int J Mol Sci. 2022. PMID: 35806020 Free PMC article.
-
Deletion of the Cold Thermoreceptor TRPM8 Increases Heat Loss and Food Intake Leading to Reduced Body Temperature and Obesity in Mice.J Neurosci. 2018 Apr 11;38(15):3643-3656. doi: 10.1523/JNEUROSCI.3002-17.2018. Epub 2018 Mar 12. J Neurosci. 2018. PMID: 29530988 Free PMC article.
-
TRP ion channels in thermosensation, thermoregulation and metabolism.Temperature (Austin). 2015 May 26;2(2):178-87. doi: 10.1080/23328940.2015.1040604. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227022 Free PMC article. Review.
References
-
- Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun 321: 219–225, 2004 - PubMed
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007 - PubMed
-
- Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 9: 9–14, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

