Investigation of ginkgo biloba leave extracts as corrosion and Oil field microorganism inhibitors
- PMID: 23651921
- PMCID: PMC3661374
- DOI: 10.1186/1752-153X-7-83
Investigation of ginkgo biloba leave extracts as corrosion and Oil field microorganism inhibitors
Abstract
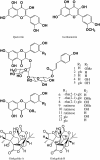
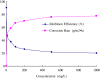
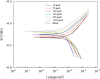
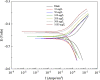
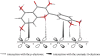
Ginkgo biloba (Ginkgoaceae), originating from China, now distributes all over the world. Wide application of Ginkgo biloba extracts is determined by the main active substances, flavonoids and terpenoids, which indicates its extracts suitable to be used as an effective corrosion inhibitor. The extracts of Ginkgo biloba leave have been investigated on the corrosion inhibition of Q235A steel with weight loss and potentiodynamic polarisation techniques. The inhibition efficiency of the extracts varies with extract concentration. The extracts inhibit corrosion mainly by adsorption mechanism. Potentiodynamic polarisation studies show that extracts are mixed type inhibitors. The antibacterial activity of the extracts against oil field microorganism (SRB, IB and TGB) was also investigated.
Figures



Similar articles
-
Investigation of Diospyros Kaki L.f husk extracts as corrosion inhibitors and bactericide in oil field.Chem Cent J. 2013 Jul 1;7(1):109. doi: 10.1186/1752-153X-7-109. Chem Cent J. 2013. PMID: 23816431 Free PMC article.
-
Identification of kaempferol as a monoamine oxidase inhibitor and potential Neuroprotectant in extracts of Ginkgo biloba leaves.J Pharm Pharmacol. 2000 Apr;52(4):451-9. doi: 10.1211/0022357001774075. J Pharm Pharmacol. 2000. PMID: 10813558
-
Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients.Clin Pharmacokinet. 2013 Sep;52(9):727-49. doi: 10.1007/s40262-013-0074-5. Clin Pharmacokinet. 2013. PMID: 23703577 Review.
-
Ginkgo biloba L. (Ginkgoaceae) Leaf Extract Medications From Different Providers Exhibit Differential Functional Effects on Mouse Frontal Cortex Neuronal Networks.Front Pharmacol. 2018 Aug 3;9:848. doi: 10.3389/fphar.2018.00848. eCollection 2018. Front Pharmacol. 2018. PMID: 30123130 Free PMC article.
-
Endophytes from Ginkgo biloba and their secondary metabolites.Chin Med. 2019 Nov 8;14:51. doi: 10.1186/s13020-019-0271-8. eCollection 2019. Chin Med. 2019. PMID: 31728156 Free PMC article. Review.
Cited by
-
Effect of Adsorption and Interactions of New Triazole-Thione-Schiff Bases on the Corrosion Rate of Carbon Steel in 1 M HCl Solution: Theoretical and Experimental Evaluation.ACS Omega. 2024 Feb 2;9(6):6761-6772. doi: 10.1021/acsomega.3c08127. eCollection 2024 Feb 13. ACS Omega. 2024. PMID: 38371797 Free PMC article.
-
Advances in the Mitigation of Microbiologically Influenced Concrete Corrosion: A Snapshot.Materials (Basel). 2024 Nov 28;17(23):5846. doi: 10.3390/ma17235846. Materials (Basel). 2024. PMID: 39685282 Free PMC article. Review.
-
Investigation of Diospyros Kaki L.f husk extracts as corrosion inhibitors and bactericide in oil field.Chem Cent J. 2013 Jul 1;7(1):109. doi: 10.1186/1752-153X-7-109. Chem Cent J. 2013. PMID: 23816431 Free PMC article.
-
Experimental and theoretical studies of Schiff bases as corrosion inhibitors.Chem Cent J. 2018 Feb 5;12(1):7. doi: 10.1186/s13065-018-0376-7. Chem Cent J. 2018. PMID: 29404816 Free PMC article.
-
Progress on pharmaceutical drugs, plant extracts and ionic liquids as corrosion inhibitors.Heliyon. 2019 Feb 1;5(2):e01143. doi: 10.1016/j.heliyon.2019.e01143. eCollection 2019 Feb. Heliyon. 2019. PMID: 30766932 Free PMC article. Review.
References
-
- Borchardt JK, Yen TF. Oil-field chemistry—enhanced recovery and production stimulation. Toronto: Ontario, Canada; 1988. pp. 608–610.
-
- Liu YR, Gao ZM, Zhang Z, Shi DF. Electrochemically aided deposition of TiO2 films on Ni-P pre-plated A3 carbon steel. Corrosion Sci Prot Technol. 2007;19:323–325.
-
- Jiang JH, Li L, Hu JX, Jiang JQ, Ma AB. New Zinc-phosphating process with RE catalyzer at low temperature for painting pretreatment of cold-rolling A3 steel sheet. Surf Technol. 2007;36(4):79–81.
-
- Sastri VS. Corrosion inhibitors principle and application. New York: John Willey & Sons; 1998. pp. 198–202.
-
- Raman A, Labine P, Quraishi MA. Reviews on corrosion inhibitor science and technology. Houstan: NACE International; 2004. 3.
LinkOut - more resources
Full Text Sources
Other Literature Sources

