Select interneuron clusters determine female sexual receptivity in Drosophila
- PMID: 23652013
- PMCID: PMC3674241
- DOI: 10.1038/ncomms2837
Select interneuron clusters determine female sexual receptivity in Drosophila
Abstract
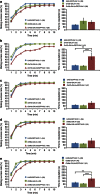
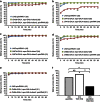
Female Drosophila with the spinster mutation repel courting males and rarely mate. Here we show that the non-copulating phenotype can be recapitulated by the elimination of spinster functions from either spin-A or spin-D neuronal clusters, in the otherwise wild-type (spinster heterozygous) female brain. Spin-D corresponds to the olfactory projection neurons with dendrites in the antennal lobe VA1v glomerulus that is fruitless-positive, sexually dimorphic and responsive to fly odour. Spin-A is a novel local neuron cluster in the suboesophageal ganglion, which is known to process contact chemical pheromone information and copulation-related signals. A slight reduction in spinster expression to a level with a minimal effect is sufficient to shut off female sexual receptivity if the dominant-negative mechanistic target of rapamycin is simultaneously expressed, although the latter manipulation alone has only a marginal effect. We propose that spin-mediated mechanistic target of rapamycin signal transduction in these neurons is essential for females to accept the courting male.
Figures
References
-
- Sander van Doorn G., Edelaar P. & Weissing F. J. On the origin of species by natural and sexual selection. Science 326, 1704–1707 (2009). - PubMed
-
- Manning A. The control of sexual receptivity in female. Drosophila Anim. Behav. 15, 239–250 (1967). - PubMed
-
- Suzuki K., Juni N. & Yamamoto D. Enhanced mate refusal in female Drosophila induced by a mutation in the spinster locus. Appl. Entomol. Zool. 32, 235–243 (1997).
-
- Lee T. & Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

