ESET histone methyltransferase is essential to hypertrophic differentiation of growth plate chondrocytes and formation of epiphyseal plates
- PMID: 23652029
- PMCID: PMC3885423
- DOI: 10.1016/j.ydbio.2013.04.031
ESET histone methyltransferase is essential to hypertrophic differentiation of growth plate chondrocytes and formation of epiphyseal plates
Abstract
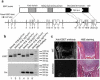
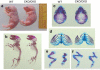
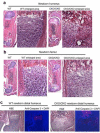
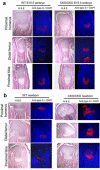
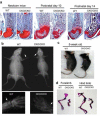
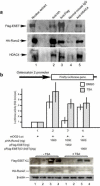
The ESET (also called SETDB1) protein contains an N-terminal tudor domain that mediates protein-protein interactions and a C-terminal SET domain that catalyzes methylation of histone H3 at lysine 9. We report here that ESET protein is transiently upregulated in prehypertrophic chondrocytes in newborn mice. To investigate the in vivo effects of ESET on chondrocyte differentiation, we generated conditional knockout mice to specifically eliminate the catalytic SET domain of ESET protein only in mesenchymal cells. Such deletion of the ESET gene caused acceleration of chondrocyte hypertrophy in both embryos and young animals, depleting chondrocytes that are otherwise available to form epiphyseal plates for endochondral bone growth. ESET-deficient mice are thus characterized by defective long bone growth and trabecular bone formation. To understand the underlying mechanism for ESET regulation of chondrocytes, we carried out co-expression experiments and found that ESET associates with histone deacetylase 4 to bind and inhibit the activity of Runx2, a hypertrophy-promoting transcription factor. Repression of Runx2-mediated gene transactivation by ESET is dependent on its H3-K9 methyltransferase activity as well as its associated histone deacetylase activity. In addition, knockout of ESET is associated with repression of Indian hedgehog gene in pre- and early hypertrophic chondrocytes. Together, these results provide clear evidence that ESET controls hypertrophic differentiation of growth plate chondrocytes and endochondral ossification during embryogenesis and postnatal development.
Published by Elsevier Inc.
Figures


Similar articles
-
Predominant expression of H3K9 methyltransferases in prehypertrophic and hypertrophic chondrocytes during mouse growth plate cartilage development.Gene Expr Patterns. 2013 Mar-Apr;13(3-4):84-90. doi: 10.1016/j.gep.2013.01.002. Epub 2013 Jan 17. Gene Expr Patterns. 2013. PMID: 23333759
-
Mesenchyme-specific knockout of ESET histone methyltransferase causes ectopic hypertrophy and terminal differentiation of articular chondrocytes.J Biol Chem. 2013 Nov 8;288(45):32119-32125. doi: 10.1074/jbc.M113.473827. Epub 2013 Sep 20. J Biol Chem. 2013. PMID: 24056368 Free PMC article.
-
Generation and characterization of mice with mesenchyme-specific deletion of the entire ESET histone methyltransferase protein.Genesis. 2018 Feb;56(2). doi: 10.1002/dvg.23088. Epub 2018 Jan 13. Genesis. 2018. PMID: 29282851
-
Transcriptional networks controlling chondrocyte proliferation and differentiation during endochondral ossification.Pediatr Nephrol. 2010 Apr;25(4):625-31. doi: 10.1007/s00467-009-1368-6. Epub 2009 Dec 1. Pediatr Nephrol. 2010. PMID: 19949815 Review.
-
Recent Insights into Long Bone Development: Central Role of Hedgehog Signaling Pathway in Regulating Growth Plate.Int J Mol Sci. 2019 Nov 20;20(23):5840. doi: 10.3390/ijms20235840. Int J Mol Sci. 2019. PMID: 31757091 Free PMC article. Review.
Cited by
-
Epigenetic regulation in chondrocyte phenotype maintenance for cell-based cartilage repair.Am J Transl Res. 2015 Nov 15;7(11):2127-40. eCollection 2015. Am J Transl Res. 2015. PMID: 26807163 Free PMC article. Review.
-
Genes uniquely expressed in human growth plate chondrocytes uncover a distinct regulatory network.BMC Genomics. 2017 Dec 20;18(1):983. doi: 10.1186/s12864-017-4378-y. BMC Genomics. 2017. PMID: 29262782 Free PMC article.
-
SETDB1 in cancer: overexpression and its therapeutic implications.Am J Cancer Res. 2021 May 15;11(5):1803-1827. eCollection 2021. Am J Cancer Res. 2021. PMID: 34094655 Free PMC article. Review.
-
[Correlation between histone methylation level and pathological development of osteoarthritis].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019 Dec 25;48(6):682-687. doi: 10.3785/j.issn.1008-9292.2019.12.14. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 31955544 Free PMC article. Review. Chinese.
-
JMJD3 promotes chondrocyte proliferation and hypertrophy during endochondral bone formation in mice.J Mol Cell Biol. 2015 Feb;7(1):23-34. doi: 10.1093/jmcb/mjv003. Epub 2015 Jan 13. J Mol Cell Biol. 2015. PMID: 25587042 Free PMC article.
References
-
- Blackburn ML, Chansky HA, Zielinska-Kwiatkowska A, Matsui Y, Yang L. Genomic structure and expression of the mouse ESET gene encoding an ERG-associated histone methyltransferase with a SET domain. Biochim Biophys Acta. 2003;1629:8–14. - PubMed
-
- Ceol CJ, Houvras Y, Jane-Valbuena J, Bilodeau S, Orlando DA, Battisti V, Fritsch L, Lin WM, Hollmann TJ, Ferre F, Bourque C, Burke CJ, Turner L, Uong A, Johnson LA, Beroukhim R, Mermel CH, Loda M, Ait-Si-Ali S, Garraway LA, Young RA, Zon LI. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–7. - PMC - PubMed
-
- Fritsch L, Robin P, Mathieu JR, Souidi M, Hinaux H, Rougeulle C, Harel-Bellan A, Ameyar-Zazoua M, Ait-Si-Ali S. A subset of the histone H3 lysine 9 methyltransferases Suv39h1, G9a, GLP, and SETDB1 participate in a multimeric complex. Mol Cell. 2010;37:46–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

