Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/μ-opioid receptor ligands in mouse models of neuropathic and inflammatory pain
- PMID: 23652222
- PMCID: PMC3684842
- DOI: 10.1124/jpet.113.203984
Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/μ-opioid receptor ligands in mouse models of neuropathic and inflammatory pain
Abstract
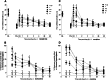
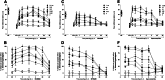
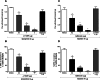
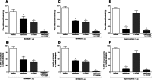
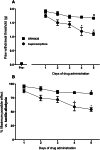
Nociceptin/orphanin FQ peptide receptor (NOP) agonists produce antinociceptive effects in animal models after spinal administration and potentiate μ-opioid receptor (MOP)-mediated antinociception. This study determined the antinociceptive effects of spinally administered bifunctional NOP/MOP ligands and the antinociceptive functions of spinal NOP and MOP receptors in mice. Antinociceptive effects of bifunctional NOP/MOP ligands BU08028 [(2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol] and SR16435 [1-(1-(2,3,3α,4,5,6-hexahydro-1H-phenalen-1-yl)piperidin-4-yl)-indolin-2-one] were pharmacologically compared with the putative bifunctional ligand buprenorphine, selective NOP agonist SCH221510 [3-endo-8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol] and selective MOP agonist morphine in neuropathic and inflammatory pain models. Additionally, the degree of tolerance development to the antiallodynic effects of SR16435 and buprenorphine were determined after repeated intrathecal administration. Our data indicated that BU08028 and SR16435 were more potent than morphine and SCH221510 in attenuating nerve injury-induced tactile allodynia and inflammation-induced thermal hyperalgesia. Coadministration of receptor-selective antagonists further revealed that both NOP and MOP in the spinal cord mediated the antiallodynic effects of BU08028 and SR16435, but intrathecal buprenorphine-induced antiallodynic effects were primarily mediated by MOP. Repeated intrathecal administration of SR16435 resulted in reduced and slower development of tolerance to its antiallodynic effects compared with buprenorphine. In conclusion, both NOP and MOP receptors in the spinal cord independently drive antinociception in mice. Spinally administered bifunctional NOP/MOP ligands not only can effectively attenuate neuropathic and inflammatory pain, but also have higher antinociceptive potency with reduced tolerance development to analgesia. Such ligands therefore display a promising profile as spinal analgesics.
Figures
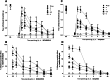


Similar articles
-
Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists.J Pharmacol Exp Ther. 2009 Dec;331(3):946-53. doi: 10.1124/jpet.109.156711. Epub 2009 Aug 27. J Pharmacol Exp Ther. 2009. PMID: 19713488 Free PMC article.
-
The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward.J Pharmacol Exp Ther. 2011 Mar;336(3):952-61. doi: 10.1124/jpet.110.175620. Epub 2010 Dec 21. J Pharmacol Exp Ther. 2011. PMID: 21177476 Free PMC article.
-
Effects of stimulation of mu opioid and nociceptin/orphanin FQ peptide (NOP) receptors on alcohol drinking in rhesus monkeys.Neuropsychopharmacology. 2019 Jul;44(8):1476-1484. doi: 10.1038/s41386-019-0390-z. Epub 2019 Apr 10. Neuropsychopharmacology. 2019. PMID: 30970376 Free PMC article.
-
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.Pharmacol Ther. 2014 Mar;141(3):283-99. doi: 10.1016/j.pharmthera.2013.10.011. Epub 2013 Nov 1. Pharmacol Ther. 2014. PMID: 24189487 Free PMC article. Review.
-
The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability.ACS Chem Neurosci. 2013 Feb 20;4(2):214-24. doi: 10.1021/cn300124f. Epub 2012 Nov 6. ACS Chem Neurosci. 2013. PMID: 23421672 Free PMC article. Review.
Cited by
-
CCR4 Antagonist (C021) Administration Diminishes Hypersensitivity and Enhances the Analgesic Potency of Morphine and Buprenorphine in a Mouse Model of Neuropathic Pain.Front Immunol. 2020 Jul 14;11:1241. doi: 10.3389/fimmu.2020.01241. eCollection 2020. Front Immunol. 2020. PMID: 32760393 Free PMC article.
-
ORL1 Activation Mediates a Novel ORL1 Receptor Agonist SCH221510 Analgesia in Neuropathic Pain in Rats.J Mol Neurosci. 2018 Sep;66(1):10-16. doi: 10.1007/s12031-018-1140-0. Epub 2018 Aug 3. J Mol Neurosci. 2018. PMID: 30074175
-
Usefulness of knockout mice to clarify the role of the opioid system in chronic pain.Br J Pharmacol. 2018 Jul;175(14):2791-2808. doi: 10.1111/bph.14088. Epub 2018 Jan 6. Br J Pharmacol. 2018. PMID: 29124744 Free PMC article. Review.
-
Buprenorphine requires concomitant activation of NOP and MOP receptors to reduce cocaine consumption.Addict Biol. 2018 Mar;23(2):585-595. doi: 10.1111/adb.12513. Epub 2017 Jun 21. Addict Biol. 2018. PMID: 28635181 Free PMC article.
-
Multifunctional Opioid-Derived Hybrids in Neuropathic Pain: Preclinical Evidence, Ideas and Challenges.Molecules. 2020 Nov 25;25(23):5520. doi: 10.3390/molecules25235520. Molecules. 2020. PMID: 33255641 Free PMC article. Review.
References
-
- Bennett GJ, Xie YK. (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107 - PubMed
-
- Bernatzky G, Jurna I. (1986) Intrathecal injection of codeine, buprenorphine, tilidine, tramadol and nefopam depresses the tail-flick response in rats. Eur J Pharmacol 120:75–80 - PubMed
-
- Briscini L, Corradini L, Ongini E, Bertorelli R. (2002) Up-regulation of ORL-1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur J Pharmacol 447:59–65 - PubMed
-
- Calo G, Guerrini R. (2013) Medicinal chemistry, pharmacology, and biological actions of peptide ligands selective for the NOP receptor, in Research and Development of Opioid-Related Analgesics (Ko MC, Husbands SM, eds) pp 275–325, ACS Books, Washington, DC
-
- Celleno D, Capogna G. (1989) Spinal buprenorphine for postoperative analgesia after caesarean section. Acta Anaesthesiol Scand 33:236–238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

