Autoantibodies to MUC1 glycopeptides cannot be used as a screening assay for early detection of breast, ovarian, lung or pancreatic cancer
- PMID: 23652307
- PMCID: PMC3670483
- DOI: 10.1038/bjc.2013.214
Autoantibodies to MUC1 glycopeptides cannot be used as a screening assay for early detection of breast, ovarian, lung or pancreatic cancer
Abstract
Background: Autoantibodies have been detected in sera before diagnosis of cancer leading to interest in their potential as screening/early detection biomarkers. As we have found autoantibodies to MUC1 glycopeptides to be elevated in early-stage breast cancer patients, in this study we analysed these autoantibodies in large population cohorts of sera taken before cancer diagnosis.
Methods: Serum samples from women who subsequently developed breast cancer, and aged-matched controls, were identified from UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) and Guernsey serum banks to formed discovery and validation sets. These were screened on a microarray platform of 60mer MUC1 glycopeptides and recombinant MUC1 containing 16 tandem repeats. Additional case-control sets comprised of women who subsequently developed ovarian, pancreatic and lung cancer were also screened on the arrays.
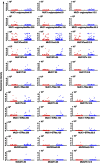
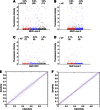
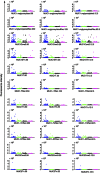
Results: In the discovery (273 cases, 273 controls) and the two validation sets (UKCTOCS 426 cases, 426 controls; Guernsey 303 cases and 606 controls), no differences were found in autoantibody reactivity to MUC1 tandem repeat peptide or glycoforms between cases and controls. Furthermore, no differences were observed between ovarian, pancreatic and lung cancer cases and controls.
Conclusion: This robust, validated study shows autoantibodies to MUC1 peptide or glycopeptides cannot be used for breast, ovarian, lung or pancreatic cancer screening. This has significant implications for research on the use of MUC1 in cancer detection.
Figures
Similar articles
-
Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis.Breast Cancer Res. 2011 Mar 8;13(2):R25. doi: 10.1186/bcr2841. Breast Cancer Res. 2011. PMID: 21385452 Free PMC article.
-
Cancer-associated autoantibodies to MUC1 and MUC4--a blinded case–control study of colorectal cancer in UK collaborative trial of ovarian cancer screening.Int J Cancer. 2014 May 1;134(9):2180-88. doi: 10.1002/ijc.28538. Int J Cancer. 2014. PMID: 24122770 Free PMC article.
-
Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes.Cancer Res. 2010 Feb 15;70(4):1306-13. doi: 10.1158/0008-5472.CAN-09-2893. Epub 2010 Feb 2. Cancer Res. 2010. PMID: 20124478 Free PMC article.
-
Tecemotide: an antigen-specific cancer immunotherapy.Hum Vaccin Immunother. 2014;10(11):3383-93. doi: 10.4161/hv.29836. Hum Vaccin Immunother. 2014. PMID: 25483673 Free PMC article. Review.
-
[Serum autoantibodies profiling and early-stage cancer detection].Med Sci (Paris). 2011 Jun-Jul;27(6-7):633-8. doi: 10.1051/medsci/2011276016. Epub 2011 Jul 1. Med Sci (Paris). 2011. PMID: 21718648 Review. French.
Cited by
-
Autoantibodies as biomarkers for breast cancer diagnosis and prognosis.Front Immunol. 2022 Nov 14;13:1035402. doi: 10.3389/fimmu.2022.1035402. eCollection 2022. Front Immunol. 2022. PMID: 36451832 Free PMC article. Review.
-
Identification of Serum Biomarkers for Gastric Cancer Diagnosis Using a Human Proteome Microarray.Mol Cell Proteomics. 2016 Feb;15(2):614-23. doi: 10.1074/mcp.M115.051250. Epub 2015 Nov 23. Mol Cell Proteomics. 2016. PMID: 26598640 Free PMC article.
-
Enhanced humoral immunity in breast cancer patients with high serum concentration of anti-HER2 autoantibody.Cancer Med. 2021 Feb;10(4):1418-1430. doi: 10.1002/cam4.3742. Epub 2021 Jan 27. Cancer Med. 2021. PMID: 33506656 Free PMC article.
-
A systematic review of serum autoantibodies as biomarkers for pancreatic cancer detection.Oncotarget. 2016 Mar 8;7(10):11151-64. doi: 10.18632/oncotarget.7098. Oncotarget. 2016. PMID: 26840568 Free PMC article.
-
The dawn of vaccines for cancer prevention.Nat Rev Immunol. 2018 Mar;18(3):183-194. doi: 10.1038/nri.2017.140. Epub 2017 Dec 27. Nat Rev Immunol. 2018. PMID: 29279613 Review.
References
-
- Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, Barnes A, Robertson J. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol. 2007;18:868–873. - PubMed
-
- Chapman CJ, Healey GF, Murray A, Boyle P, Robertson C, Peek LJ, Allen J, Thorpe AJ, Hamilton-Fairley G, Parsy-Kowalska CB, Macdonald IK, Jewell W, Maddison P, Robertson JF. EarlyCDT-Lung test: improved clinical utility through additional autoantibody assays. Tumour Biol. 2012;33:1319–1226. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

