Enhanced inhibitory neurotransmission in the cerebellar cortex of Atp1a3-deficient heterozygous mice
- PMID: 23652595
- PMCID: PMC3717237
- DOI: 10.1113/jphysiol.2012.247817
Enhanced inhibitory neurotransmission in the cerebellar cortex of Atp1a3-deficient heterozygous mice
Abstract
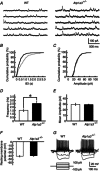
Dystonia is characterized by excessive involuntary and prolonged simultaneous contractions of both agonist and antagonist muscles. Although the basal ganglia have long been proposed as the primary region, recent studies indicated that the cerebellum also plays a key role in the expression of dystonia. One hereditary form of dystonia, rapid-onset dystonia with parkinsonism (RDP), is caused by loss of function mutations of the gene for the Na pump α3 subunit (ATP1A3). Little information is available on the affected brain regions and mechanism for dystonia by the mutations in RDP. The Na pump is composed of α and β subunits and maintains ionic gradients of Na(+) and K(+) across the cell membrane. The gradients are utilized for neurotransmitter reuptake and their alteration modulates neural excitability. To provide insight into the molecular aetiology of RDP, we generated and analysed knockout heterozygous mice (Atp1a3(+/-)). Atp1a3(+/-) showed increased symptoms of dystonia that is induced by kainate injection into the cerebellar vermis. Atp1a3 mRNA was highly expressed in Purkinje cells and molecular-layer interneurons, and its product was concentrated at Purkinje cell soma, the site of abundant vesicular γ-aminobutyric acid transporter (VGAT) signal, suggesting the presynaptic localization of the α3 subunit in the inhibitory synapse. Electrophysiological studies showed that the inhibitory neurotransmission at molecular-layer interneuron-Purkinje cell synapses was enhanced in Atp1a3(+/-) cerebellar cortex, and that the enhancement originated via a presynaptic mechanism. Our results shed light on the role of Atp1a3 in the inhibitory synapse, and potential involvement of inhibitory synaptic dysfunction for the pathophysiology of dystonia.
Figures





References
-
- Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre–Purkinje cell pathway in rat cerebellar slices. Neuroscience. 1994;63:911–915. - PubMed
-
- Blanco-Arias P, Einholm AP, Mamsa H, Concheiro C, Gutiérrez-de-Terán H, Romero J, Toustrup-Jensen MS, Carracedo Á, Jen JC, Vilsen B, Sobrido MJ. A C-terminal mutation of ATP1A3 underscores the crucial role of sodium affinity in the pathophysiology of rapid-onset dystonia-parkinsonism. Hum Mol Genet. 2009;18:2370–2377. - PubMed
-
- Blanco G. The Na/K-ATPase and its isozymes: what we have learned using the baculovirus expression system. Front Biosci. 2005;10:2397–2411. - PubMed
-
- Bøttger P, Tracz Z, Heuck A, Nissen P, Romero-Ramos M, Lykke-Hartmann K. Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J Comp Neurol. 2011;519:376–404. - PubMed
-
- Brashear A, Dobyns WB, de Carvalho Aguiar P, Borg M, Frijns CJM, Gollamudi S, Green A, Guimaraes J, Haake BC, Klein C, Linazasoro G, Münchau A, Raymond D, Riley D, Saunders-Pullman R, Tijssen MAJ, Webb D, Zaremba J, Bressman SB, Ozelius LJ. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130:828–835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

