Modeling of brain metabolism and pyruvate compartmentation using (13)C NMR in vivo: caution required
- PMID: 23652627
- PMCID: PMC3734769
- DOI: 10.1038/jcbfm.2013.67
Modeling of brain metabolism and pyruvate compartmentation using (13)C NMR in vivo: caution required
Abstract
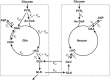
Two variants of a widely used two-compartment model were prepared for fitting the time course of [1,6-(13)C2]glucose metabolism in rat brain. Features common to most models were included, but in one model the enrichment of the substrates entering the glia and neuronal citric acid cycles was allowed to differ. Furthermore, the models included the capacity to analyze multiplets arising from (13)C spin-spin coupling, known to improve parameter estimates in heart. Data analyzed were from a literature report providing time courses of [1,6-(13)C2]glucose metabolism. Four analyses were used, two comparing the effect of different pyruvate enrichment in glia and neurons, and two for determining the effect of multiplets present in the data. When fit independently, the enrichment in glial pyruvate was less than in neurons. In the absence of multiplets, fit quality and parameter values were typical of those in the literature, whereas the multiplet curves were not modeled well. This prompted the use of robust statistical analysis (the Kolmogorov-Smirnov test of goodness of fit) to determine whether individual curves were modeled appropriately. At least 50% of the curves in each experiment were considered poorly fit. It was concluded that the model does not include all metabolic features required to analyze the data.
Figures


References
-
- Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro13C NMR. J Biol Chem. 1990;265:12916–12926. - PubMed
-
- Henry PG, Oz G, Provencher S, Gruetter R. Toward dynamic isotopomer analysis in the rat brain in vivo: automatic quantitation of 13C NMR spectra using LCModel. NMR Biomed. 2003;16:400–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL034557/HL/NHLBI NIH HHS/United States
- R37 HL034557/HL/NHLBI NIH HHS/United States
- 5R37HL034557-24/HL/NHLBI NIH HHS/United States
- R01 EB000461/EB/NIBIB NIH HHS/United States
- F32 NS065640/NS/NINDS NIH HHS/United States
- F32NS065640/NS/NINDS NIH HHS/United States
- R01NS38672/NS/NINDS NIH HHS/United States
- P41 RR002584/RR/NCRR NIH HHS/United States
- P41 EB015894/EB/NIBIB NIH HHS/United States
- P41 RR008079/RR/NCRR NIH HHS/United States
- P41RR008079/RR/NCRR NIH HHS/United States
- R01 NS077015/NS/NINDS NIH HHS/United States
- RR002584/RR/NCRR NIH HHS/United States
- R01 NS038672/NS/NINDS NIH HHS/United States
- EB000461/EB/NIBIB NIH HHS/United States
- NS077115/NS/NINDS NIH HHS/United States
- P41EB015894/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

