Vectofusin-1, a new viral entry enhancer, strongly promotes lentiviral transduction of human hematopoietic stem cells
- PMID: 23653154
- PMCID: PMC4817938
- DOI: 10.1038/mtna.2013.17
Vectofusin-1, a new viral entry enhancer, strongly promotes lentiviral transduction of human hematopoietic stem cells
Abstract
Gene transfer into hCD34(+) hematopoietic stem/progenitor cells (HSCs) using human immunodeficiency virus type 1 (HIV-1)-based lentiviral vectors (LVs) has several promising therapeutic applications. Yet, efficiency, safety, and cost of LV gene therapy could be ameliorated by enhancing target cell transduction levels and reducing the amount of LV used on the cells. Several transduction enhancers already exist such as fibronectin fragments and cationic compounds, but all present limitations. In this study, we describe a new transduction enhancer called Vectofusin-1, which is a short cationic peptide, active on several LV pseudotypes. Vectofusin-1 is used as a soluble additive to safely increase the frequency of transduced HSCs and to augment the level of transduction to one or two copies of vector per cell in a vector dose-dependent manner. Vectofusin-1 acts at the entry step by promoting the adhesion and the fusion between viral and cellular membranes. Vectofusin-1 is therefore a promising additive that could significantly ameliorate hCD34(+) cell-based gene therapy.Molecular Therapy-Nucleic Acids (2013) 2, e90; doi:10.1038/mtna.2013.17; published online 7 May 2013.
Figures

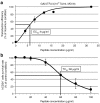
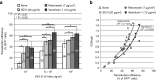



References
-
- D'Costa, J, Mansfield, SG and Humeau, LM (2009). Lentiviral vectors in clinical trials: Current status. Curr Opin Mol Ther 11: 554–564. - PubMed
-
- Naldini, L (2011). Ex vivo gene transfer and correction for cell-based therapies. Nat Rev Genet 12: 301–315. - PubMed
-
- Merten, OW, Charrier, S, Laroudie, N, Fauchille, S, Dugué, C, Jenny, C et al. (2011). Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum Gene Ther 22: 343–356. - PubMed
-
- Sandrin, V, Boson, B, Salmon, P, Gay, W, Nègre, D, Le Grand, R et al. (2002). Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood 100: 823–832. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

