Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1
- PMID: 23653359
- PMCID: PMC3682571
- DOI: 10.1074/jbc.M112.445403
Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1
Abstract
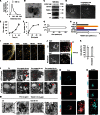
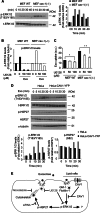
The role of exosomes in cancer can be inferred from the observation that they transfer tumor cell derived genetic material and signaling proteins, resulting in e.g. increased tumor angiogenesis and metastasis. However, the membrane transport mechanisms and the signaling events involved in the uptake of these virus-like particles remain ill-defined. We now report that internalization of exosomes derived from glioblastoma (GBM) cells involves nonclassical, lipid raft-dependent endocytosis. Importantly, we show that the lipid raft-associated protein caveolin-1 (CAV1), in analogy with its previously described role in virus uptake, negatively regulates the uptake of exosomes. We find that exosomes induce the phosphorylation of several downstream targets known to associate with lipid rafts as signaling and sorting platforms, such as extracellular signal-regulated kinase-1/2 (ERK1/2) and heat shock protein 27 (HSP27). Interestingly, exosome uptake appears dependent on unperturbed ERK1/2-HSP27 signaling, and ERK1/2 phosphorylation is under negative influence by CAV1 during internalization of exosomes. These findings significantly advance our general understanding of exosome-mediated uptake and offer potential strategies for how this pathway may be targeted through modulation of CAV1 expression and ERK1/2 signaling.
Keywords: Caveolin; Endocytosis; Exosomes; Glioblastoma; Microvesicles; Vesicles.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

