Progesterone antagonism of neurite outgrowth depends on microglial activation via Pgrmc1/S2R
- PMID: 23653459
- PMCID: PMC3689281
- DOI: 10.1210/en.2012-2109
Progesterone antagonism of neurite outgrowth depends on microglial activation via Pgrmc1/S2R
Abstract
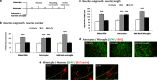
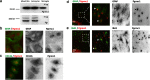
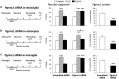
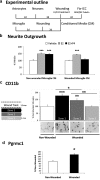
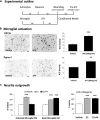
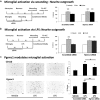
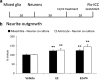
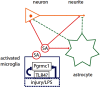
Neuronal plasticity is regulated by the ovarian steroids estradiol (E2) and progesterone (P4) in many normal brain functions, as well as in acute response to injury and chronic neurodegenerative disease. In a female rat model of axotomy, the E2-dependent compensatory neuronal sprouting is antagonized by P4. To resolve complex glial-neuronal cell interactions, we used the "wounding-in-a-dish" model of neurons cocultured with astrocytes or mixed glia (microglia to astrocytes, 1:3). Although both astrocytes and mixed glia supported E2-enhanced neurite outgrowth, P4 antagonized E2-induced neurite outgrowth only with mixed glia, but not astrocytes alone. We now show that P4-E2 antagonism of neurite outgrowth is mediated by microglial expression of progesterone receptor (Pgr) membrane component 1 (Pgrmc1)/S2R, a putative nonclassical Pgr mediator with multiple functions. The P4-E2 antagonism of neurite outgrowth was restored by add-back of microglia to astrocyte-neuron cocultures. Because microglia do not express the classical Pgr, we examined the role of Pgrmc1, which is expressed in microglia in vitro and in vivo. Knockdown by siRNA-Pgrmc1 in microglia before add-back to astrocyte-neuron cocultures suppressed the P4-E2 antagonism of neurite outgrowth. Conditioned media from microglia restored the P4-E2 activity, but only if microglia were activated by lipopolysaccharide or by wounding. Moreover, the microglial activation was blocked by Pgmrc1-siRNA knockdown. These findings explain why nonwounded cultures without microglial activation lack P4 antagonism of E2-induced neurite outgrowth. We suggest that microglial activation may influence brain responses to exogenous P4, which is a prospective therapy in traumatic brain injury.
Figures
References
-
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306 - PubMed
-
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

