Collagen scaffolds in bone sialoprotein-mediated bone regeneration
- PMID: 23653530
- PMCID: PMC3628497
- DOI: 10.1155/2013/812718
Collagen scaffolds in bone sialoprotein-mediated bone regeneration
Abstract
Decades of research in bioengineering have resulted in the development of many types of 3-dimentional (3D) scaffolds for use as drug delivery systems (DDS) and for tissue regeneration. Scaffolds may be comprised of different natural fibers and synthetic polymers as well as ceramics in order to exert the most beneficial attributes including biocompatibility, biodegradability, structural integrity, cell infiltration and attachment, and neovascularization. Type I collagen scaffolds meet most of these criteria. In addition, type I collagen binds integrins through RGD and non-RGD sites which facilitates cell migration, attachment, and proliferation. Type I collagen scaffolds can be used for bone tissue repair when they are coated with osteogenic proteins such as bone morphogenic protein (BMP) and bone sialoprotein (BSP). BSP, a small integrin-binding ligand N-linked glycoprotein (SIBLING), has osteogenic properties and plays an essential role in bone formation. BSP also mediates mineral deposition, binds type I collagen with high affinity, and binds α v β 3 and α v β 5 integrins which mediate cell signaling. This paper reviews the emerging evidence demonstrating the efficacy of BSP-collagen scaffolds in bone regeneration.
Figures
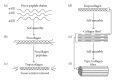
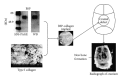
References
-
- Di Lullo GA, Sweeney SM, Körkkö J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. The Journal of Biological Chemistry. 2002;277(6):4223–4231. - PubMed
-
- Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. Journal of Cell Science. 2007;120, part 12:1955–1958. - PubMed
-
- Veit G, Kobbe B, Keene DR, Paulsson M, Koch M, Wagener R. Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. The Journal of Biological Chemistry. 2006;281(6):3494–3504. - PubMed
-
- Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Advanced Drug Delivery Reviews. 2003;55(12):1531–1546. - PubMed
-
- Huang S, Fu X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. Journal of Controlled Release. 2010;142(2):149–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

