Optogenetic elevation of endogenous glucocorticoid level in larval zebrafish
- PMID: 23653595
- PMCID: PMC3644639
- DOI: 10.3389/fncir.2013.00082
Optogenetic elevation of endogenous glucocorticoid level in larval zebrafish
Abstract
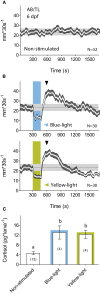
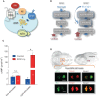
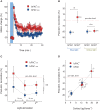
The stress response is a suite of physiological and behavioral processes that help to maintain or reestablish homeostasis. Central to the stress response is the hypothalamic-pituitary-adrenal (HPA) axis, as it releases crucial hormones in response to stress. Glucocorticoids (GCs) are the final effector hormones of the HPA axis, and exert a variety of actions under both basal and stress conditions. Despite their far-reaching importance for health, specific GC effects have been difficult to pin-down due to a lack of methods for selectively manipulating endogenous GC levels. Hence, in order to study stress-induced GC effects, we developed a novel optogenetic approach to selectively manipulate the rise of GCs triggered by stress. Using this approach, we could induce both transient hypercortisolic states and persistent forms of hypercortisolaemia in freely behaving larval zebrafish. Our results also established that transient hypercortisolism leads to enhanced locomotion shortly after stressor exposure. Altogether, we present a highly specific method for manipulating the gain of the stress axis with high temporal accuracy, altering endocrine and behavioral responses to stress as well as basal GC levels. Our study offers a powerful tool for the analysis of rapid (non-genomic) and delayed (genomic) GC effects on brain function and behavior, feedbacks within the stress axis and developmental programming by GCs.
Keywords: HPA axis; glucocorticoids; larval zebrafish; optogenetics; stress response.
Figures



Similar articles
-
Optogenetic induction of chronic glucocorticoid exposure in early-life leads to blunted stress-response in larval zebrafish.Eur J Neurosci. 2024 Jun;59(11):3134-3146. doi: 10.1111/ejn.16301. Epub 2024 Apr 11. Eur J Neurosci. 2024. PMID: 38602078
-
Behavioral and physiological indicators of stress coping styles in larval zebrafish.Stress. 2015 Jan;18(1):121-8. doi: 10.3109/10253890.2014.989205. Epub 2015 Jan 8. Stress. 2015. PMID: 25407298
-
Altered glucocorticoid reactivity and behavioral phenotype in rx3-/- larval zebrafish.Front Endocrinol (Lausanne). 2023 Jul 6;14:1187327. doi: 10.3389/fendo.2023.1187327. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37484970 Free PMC article.
-
Contribution of glucocorticoids and glucocorticoid receptors to the regulation of neurodegenerative processes.CNS Neurol Disord Drug Targets. 2013 Dec;12(8):1175-93. CNS Neurol Disord Drug Targets. 2013. PMID: 24040816 Review.
-
Chronic Stress and Glucocorticoid Receptor Resistance in Asthma.Clin Ther. 2020 Jun;42(6):993-1006. doi: 10.1016/j.clinthera.2020.03.002. Epub 2020 Mar 26. Clin Ther. 2020. PMID: 32224031 Review.
Cited by
-
Positive taxis and sustained responsiveness to water motions in larval zebrafish.Front Neural Circuits. 2015 Mar 6;9:9. doi: 10.3389/fncir.2015.00009. eCollection 2015. Front Neural Circuits. 2015. PMID: 25798089 Free PMC article.
-
The Effects of Early Life Stress on the Brain and Behaviour: Insights From Zebrafish Models.Front Cell Dev Biol. 2021 Jul 21;9:657591. doi: 10.3389/fcell.2021.657591. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34368117 Free PMC article. Review.
-
Optimizing optogenetic constructs for control over signaling and cell behaviours.Photochem Photobiol Sci. 2015 Sep 26;14(9):1578-85. doi: 10.1039/c5pp00171d. Epub 2015 Jul 2. Photochem Photobiol Sci. 2015. PMID: 26135203 Free PMC article. Review.
-
Early-life glucocorticoids programme behaviour and metabolism in adulthood in zebrafish.J Endocrinol. 2016 Jul;230(1):125-42. doi: 10.1530/JOE-15-0376. J Endocrinol. 2016. PMID: 27390302 Free PMC article.
-
Investigating neuronal function with optically controllable proteins.Front Mol Neurosci. 2015 Jul 21;8:37. doi: 10.3389/fnmol.2015.00037. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26257603 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

