Common muscle synergies for balance and walking
- PMID: 23653605
- PMCID: PMC3641709
- DOI: 10.3389/fncom.2013.00048
Common muscle synergies for balance and walking
Abstract
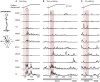
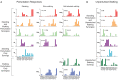
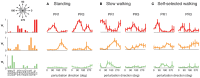
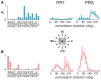
Little is known about the integration of neural mechanisms for balance and locomotion. Muscle synergies have been studied independently in standing balance and walking, but not compared. Here, we hypothesized that reactive balance and walking are mediated by a common set of lower-limb muscle synergies. In humans, we examined muscle activity during multidirectional support-surface perturbations during standing and walking, as well as unperturbed walking at two speeds. We show that most muscle synergies used in perturbations responses during standing were also used in perturbation responses during walking, suggesting common neural mechanisms for reactive balance across different contexts. We also show that most muscle synergies using in reactive balance were also used during unperturbed walking, suggesting that neural circuits mediating locomotion and reactive balance recruit a common set of muscle synergies to achieve task-level goals. Differences in muscle synergies across conditions reflected differences in the biomechanical demands of the tasks. For example, muscle synergies specific to walking perturbations may reflect biomechanical challenges associated with single limb stance, and muscle synergies used during sagittal balance recovery in standing but not walking were consistent with maintaining the different desired center of mass motions in standing vs. walking. Thus, muscle synergies specifying spatial organization of muscle activation patterns may define a repertoire of biomechanical subtasks available to different neural circuits governing walking and reactive balance and may be recruited based on task-level goals. Muscle synergy analysis may aid in dissociating deficits in spatial vs. temporal organization of muscle activity in motor deficits. Muscle synergy analysis may also provide a more generalizable assessment of motor function by identifying whether common modular mechanisms are impaired across the performance of multiple motor tasks.
Keywords: electromyography; locomotion; motor control; muscle synergy; posture.
Figures


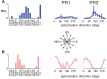
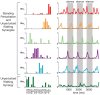
Similar articles
-
Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts.J Neurophysiol. 2010 Jun;103(6):3084-98. doi: 10.1152/jn.00960.2009. Epub 2010 Apr 14. J Neurophysiol. 2010. PMID: 20393070 Free PMC article.
-
Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking.J Neurosci. 2012 Aug 29;32(35):12237-50. doi: 10.1523/JNEUROSCI.6344-11.2012. J Neurosci. 2012. PMID: 22933805 Free PMC article.
-
Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies.J Neurophysiol. 2013 Jan;109(1):31-45. doi: 10.1152/jn.00684.2012. Epub 2012 Oct 24. J Neurophysiol. 2013. PMID: 23100133 Free PMC article.
-
Review of balance recovery in response to external perturbations during daily activities.Hum Mov Sci. 2020 Feb;69:102546. doi: 10.1016/j.humov.2019.102546. Epub 2019 Dec 31. Hum Mov Sci. 2020. PMID: 31989948 Review.
-
Lower limb muscle synergies during walking after stroke: a systematic review.Disabil Rehabil. 2020 Oct;42(20):2836-2845. doi: 10.1080/09638288.2019.1578421. Epub 2019 Mar 23. Disabil Rehabil. 2020. PMID: 30905215
Cited by
-
Kinematic-Muscular Synergies Describe Human Locomotion with a Set of Functional Synergies.Biomimetics (Basel). 2024 Oct 13;9(10):619. doi: 10.3390/biomimetics9100619. Biomimetics (Basel). 2024. PMID: 39451826 Free PMC article.
-
Patterns of whole-body muscle activations following vertical perturbations during standing and walking.J Neuroeng Rehabil. 2021 May 6;18(1):75. doi: 10.1186/s12984-021-00836-0. J Neuroeng Rehabil. 2021. PMID: 33957953 Free PMC article.
-
Plasma Aβ and neurofilament light chain are associated with cognitive and physical function decline in non-dementia older adults.Alzheimers Res Ther. 2020 Oct 8;12(1):128. doi: 10.1186/s13195-020-00697-0. Alzheimers Res Ther. 2020. PMID: 33032662 Free PMC article.
-
Muscle Synergies During Repetitive Stoop Lifting With a Bioelectrically-Controlled Lumbar Support Exoskeleton.Front Hum Neurosci. 2019 Apr 30;13:142. doi: 10.3389/fnhum.2019.00142. eCollection 2019. Front Hum Neurosci. 2019. PMID: 31114492 Free PMC article.
-
Improvement in gait stability in older adults after ten sessions of standing balance training.PLoS One. 2022 Jul 27;17(7):e0242115. doi: 10.1371/journal.pone.0242115. eCollection 2022. PLoS One. 2022. PMID: 35895709 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

