LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: a randomized controlled trial
- PMID: 23653829
- PMCID: PMC3644688
- DOI: 10.3802/jgo.2013.24.2.128
LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: a randomized controlled trial
Abstract
Objective: To compare the efficacy of the levonorgestrel-releasing intrauterine system (LNG-IUS) and oral norethisterone acetate (NET) for treatment of non-atypical endometrial hyperplasia in perimenopausal women.
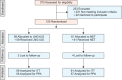
Methods: One hundred and twenty perimenopausal women with non-atypical endometrial hyperplasia were selected in this randomized controlled trial. Patients received LNG-IUS (n=59) or NET (n=61; 15 mg/day for 3 weeks/cycle) for 3-6 months. Outpatient follow-up with endometrial biopsies were undertaken at 3, 6, and 12 months intervals after treatment. Outcome measures were; the regression rate, the time to regression and hysterectomy rate.
Results: A significantly higher regression rate was noted in the LNG-IUS group than in NET group at the 3rd, 6th and 12th month follow-up visits using intention-to-treat analysis (67.8% vs. 47.5%, relative risk [RR], 1.42; 79.7% vs. 60.7%, RR, 1.31; and 88.1% vs. 55.7%, RR, 1.58, respectively). However, no significant difference was found regarding the median time to regression (3 months). The hysterectomy rate during the follow-up period was significantly higher in the NET group (57.4% vs.22%, p<0.001).
Conclusion: LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women is more effective than NET for achieving disease regression for the majority within 1 year. Moreover, it can reduce the number of hysterectomies performed.
Keywords: Abnormal uterine bleeding; Endometrial hyperplasia; Levonorgestrel-releasing intrauterine system; Non-atypical; Norethisterone acetate.
Conflict of interest statement
No potential conflict of interests relevant to this article was reported.
Figures
References
-
- Gultekin M, Dogan NU, Aksan G, Ozgul N. Management of endometrial hyperplasia. Minerva Ginecol. 2010;62:433–445. - PubMed
-
- Anastasiadis PG, Skaphida PG, Koutlaki NG, Galazios GC, Tsikouras PN, Liberis VA. Descriptive epidemiology of endometrial hyperplasia in patients with abnormal uterine bleeding. Eur J Gynaecol Oncol. 2000;21:131–134. - PubMed
-
- Espindola D, Kennedy KA, Fischer EG. Management of abnormal uterine bleeding and the pathology of endometrial hyperplasia. Obstet Gynecol Clin North Am. 2007;34:717–737. - PubMed
-
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia: a long-term study of "untreated" hyperplasia in 170 patients. Cancer. 1985;56:403–412. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources