Devices for cell transplantation into the central nervous system: Design considerations and emerging technologies
- PMID: 23653887
- PMCID: PMC3642746
- DOI: 10.4103/2152-7806.109190
Devices for cell transplantation into the central nervous system: Design considerations and emerging technologies
Abstract
Successful use of cell-based therapies for the treatment of neurological diseases is dependent upon effective delivery to the central nervous system (CNS). The CNS poses several challenges to the delivery of cell-based therapeutics, including the blood-brain barrier, anatomic complexity, and regional specificity. Targeted delivery methods are therefore required for the selective treatment of specific CNS regions. In addition, CNS tissues are mechanically and physiologically delicate and even minor injury to normal brain or spinal cord can cause devastating neurological deficits. Targeted delivery methods must therefore minimize tissue trauma. At present, direct injection into brain or spinal cord parenchyma promises to be the most versatile and accurate method of targeted CNS therapeutic delivery. While direct injection methods have already been employed in clinical trials of cell transplantation for a wide variety of neurological diseases, there are many shortcomings with the devices and surgical approaches currently used. Some of these technical limitations may hinder the clinical development of cell transplantation therapies despite validity of the underlying biological mechanisms. In this review, we discuss some of the important technical considerations of CNS injection devices such as targeting accuracy, distribution of infused therapeutic, and overall safety to the patient. We also introduce and discuss an emerging technology - radially branched deployment - that may improve our ability to safely distribute cell-based therapies and other therapeutic agents to the CNS. Finally, we speculate on future technological developments that may further enhance the efficacy of CNS therapeutic delivery.
Keywords: Cell transplantation; Parkinson's disease; radially branched deployment; stereotactic injection.
Figures




Similar articles
-
Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review.J Control Release. 2021 Jan 10;329:16-35. doi: 10.1016/j.jconrel.2020.11.049. Epub 2020 Nov 28. J Control Release. 2021. PMID: 33259851 Review.
-
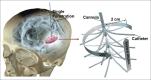
Radially branched deployment for more efficient cell transplantation at the scale of the human brain.Stereotact Funct Neurosurg. 2013;91(2):92-103. doi: 10.1159/000343213. Epub 2013 Jan 22. Stereotact Funct Neurosurg. 2013. PMID: 23343609 Free PMC article.
-
Recent Trends in Nanotechnology Toward CNS Diseases: Lipid-Based Nanoparticles and Exosomes for Targeted Therapeutic Delivery.Int Rev Neurobiol. 2016;130:1-40. doi: 10.1016/bs.irn.2016.05.002. Epub 2016 Jun 21. Int Rev Neurobiol. 2016. PMID: 27678173 Review.
-
Current strategies for therapeutic drug delivery after traumatic CNS injury.Ther Deliv. 2019 Apr;10(4):251-263. doi: 10.4155/tde-2019-0006. Ther Deliv. 2019. PMID: 30991923 Review.
-
Drug delivery to the central nervous system: a review.J Pharm Pharm Sci. 2003 May-Aug;6(2):252-73. J Pharm Pharm Sci. 2003. PMID: 12935438 Review.
Cited by
-
Dose-response relationship of MSCs as living Bio-drugs in HFrEF patients: a systematic review and meta-analysis of RCTs.Stem Cell Res Ther. 2024 Jun 13;15(1):165. doi: 10.1186/s13287-024-03713-4. Stem Cell Res Ther. 2024. PMID: 38867306 Free PMC article.
-
Preclinical development of an automated injection device for intradermal delivery of a cell-based therapy.Drug Deliv Transl Res. 2017 Oct;7(5):695-708. doi: 10.1007/s13346-017-0418-z. Drug Deliv Transl Res. 2017. PMID: 28812281 Free PMC article.
-
Early Changes in Porcine Larynges Following Injection of Motor-Endplate Expressing Muscle Cells for the Treatment of Unilateral Vocal Fold Paralysis.Laryngoscope. 2024 Jan;134(1):272-282. doi: 10.1002/lary.30868. Epub 2023 Jul 12. Laryngoscope. 2024. PMID: 37436167 Free PMC article.
-
Do foetal transplant studies continue to be justified in Huntington's disease?Neuronal Signal. 2021 Dec 13;5(4):NS20210019. doi: 10.1042/NS20210019. eCollection 2021 Dec. Neuronal Signal. 2021. PMID: 34956650 Free PMC article. Review.
-
Use of genetically modified mesenchymal stem cells to treat neurodegenerative diseases.Int J Mol Sci. 2014 Jan 23;15(2):1719-45. doi: 10.3390/ijms15021719. Int J Mol Sci. 2014. PMID: 24463293 Free PMC article. Review.
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: Current state of the art and the need for a Rosetta stone. Neuron. 2011;70:597–613. - PubMed
-
- Álvarez-Dolado M, Pardal R, García-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

