Defective endochondral ossification-derived matrix and bone cells alter the lymphopoietic niche in collagen X mouse models
- PMID: 23656481
- PMCID: PMC3780309
- DOI: 10.1089/scd.2012.0387
Defective endochondral ossification-derived matrix and bone cells alter the lymphopoietic niche in collagen X mouse models
Abstract
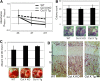
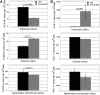
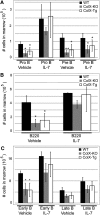
Despite the appreciated interdependence of skeletal and hematopoietic development, the cell and matrix components of the hematopoietic niche remain to be fully defined. Utilizing mice with disrupted function of collagen X (ColX), a major hypertrophic cartilage matrix protein associated with endochondral ossification, our data identified a cytokine defect in trabecular bone cells at the chondro-osseous hematopoietic niche as a cause for aberrant B lymphopoiesis in these mice. Specifically, analysis of ColX transgenic and null mouse chondro-osseous regions via micro-computed tomography revealed an altered trabecular bone environment. Additionally, cocultures with hematopoietic and chondro-osseous cell types highlighted impaired hematopoietic support by ColX transgenic and null mouse derived trabecular bone cells. Further, cytokine arrays with conditioned media from the trabecular osteoblast cocultures suggested an aberrant hematopoietic cytokine milieu within the chondro-osseous niche of the ColX deficient mice. Accordingly, B lymphopoiesis was rescued in the ColX mouse derived trabecular osteoblast cocultures with interlukin-7, stem cell factor, and stromal derived factor-1 supplementation. Moreover, B cell development was restored in vivo after injections of interlukin-7. These data support our hypothesis that endrochondrally-derived trabecular bone cells and matrix constituents provide cytokine-rich niches for hematopoiesis. Furthermore, this study contributes to the emerging concept that niche defects may underlie certain immuno-osseous and hematopoietic disorders.
Figures



Similar articles
-
Altered matrix at the chondro-osseous junction leads to defects in lymphopoiesis.Ann N Y Acad Sci. 2011 Nov;1237:79-87. doi: 10.1111/j.1749-6632.2011.06227.x. Ann N Y Acad Sci. 2011. PMID: 22082369 Review.
-
Altered endochondral ossification in collagen X mouse models leads to impaired immune responses.Dev Dyn. 2008 Oct;237(10):2693-704. doi: 10.1002/dvdy.21594. Dev Dyn. 2008. PMID: 18629872 Free PMC article.
-
Congenic mice confirm that collagen X is required for proper hematopoietic development.PLoS One. 2010 Mar 3;5(3):e9518. doi: 10.1371/journal.pone.0009518. PLoS One. 2010. PMID: 20209091 Free PMC article.
-
Altered hematopoiesis in glypican-3-deficient mice results in decreased osteoclast differentiation and a delay in endochondral ossification.Dev Biol. 2005 Jun 1;282(1):152-62. doi: 10.1016/j.ydbio.2005.03.003. Dev Biol. 2005. PMID: 15936336
-
[Development, physiology, and cell activity of bone].Ned Tijdschr Tandheelkd. 2005 Jul;112(7):258-63. Ned Tijdschr Tandheelkd. 2005. PMID: 16047964 Review. Dutch.
Cited by
-
The extracellular matrix of hematopoietic stem cell niches.Adv Drug Deliv Rev. 2022 Feb;181:114069. doi: 10.1016/j.addr.2021.114069. Epub 2021 Nov 25. Adv Drug Deliv Rev. 2022. PMID: 34838648 Free PMC article. Review.
-
Mesenchymal lineage cells and their importance in B lymphocyte niches.Bone. 2019 Feb;119:42-56. doi: 10.1016/j.bone.2017.11.018. Epub 2017 Nov 26. Bone. 2019. PMID: 29183783 Free PMC article. Review.
-
Klotho deficiency disrupts hematopoietic stem cell development and erythropoiesis.Am J Pathol. 2014 Mar;184(3):827-41. doi: 10.1016/j.ajpath.2013.11.016. Epub 2014 Jan 8. Am J Pathol. 2014. PMID: 24412515 Free PMC article.
-
Collagen remodeling-mediated signaling pathways and their impact on tumor therapy.J Biol Chem. 2025 Mar;301(3):108330. doi: 10.1016/j.jbc.2025.108330. Epub 2025 Feb 19. J Biol Chem. 2025. PMID: 39984051 Free PMC article. Review.
-
[Role of cartilage extracellular matrix for the development and function of the immune system].Z Rheumatol. 2015 Oct;74(8):711-3. doi: 10.1007/s00393-015-1650-x. Z Rheumatol. 2015. PMID: 26450435 German. No abstract available.
References
-
- Aguila HL. Rowe DW. Skeletal development, bone remodeling, and hematopoiesis. Immunol Rev. 2005;208:7–18. - PubMed
-
- Caplan AI. Cartilage begets bone versus endochondral myelopoiesis. Clin Orthop Relat Res. 1990:257–267. - PubMed
-
- Oh IH. Kwon KR. Concise review: multiple niches for hematopoietic stem cell regulations. Stem Cells. 2010;28:1243–1249. - PubMed
-
- Puron LE. Scadden DT. The hematopoietic stem cell niche. In: Silberstein L, editor. Harvard Stemcell Institute; Cambridge, MA: 2008. pp. 1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
