Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ
- PMID: 23656586
- PMCID: PMC3749068
- DOI: 10.1056/NEJMoa1213507
Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ
Abstract
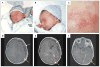
Background: The Sturge-Weber syndrome is a sporadic congenital neurocutaneous disorder characterized by a port-wine stain affecting the skin in the distribution of the ophthalmic branch of the trigeminal nerve, abnormal capillary venous vessels in the leptomeninges of the brain and choroid, glaucoma, seizures, stroke, and intellectual disability. It has been hypothesized that somatic mosaic mutations disrupting vascular development cause both the Sturge-Weber syndrome and port-wine stains, and the severity and extent of presentation are determined by the developmental time point at which the mutations occurred. To date, no such mutation has been identified.
Methods: We performed whole-genome sequencing of DNA from paired samples of visibly affected and normal tissue from 3 persons with the Sturge-Weber syndrome. We tested for the presence of a somatic mosaic mutation in 97 samples from 50 persons with the Sturge-Weber syndrome, a port-wine stain, or neither (controls), using amplicon sequencing and SNaPshot assays, and investigated the effects of the mutation on downstream signaling, using phosphorylation-specific antibodies for relevant effectors and a luciferase reporter assay.
Results: We identified a nonsynonymous single-nucleotide variant (c.548G→A, p.Arg183Gln) in GNAQ in samples of affected tissue from 88% of the participants (23 of 26) with the Sturge-Weber syndrome and from 92% of the participants (12 of 13) with apparently nonsyndromic port-wine stains, but not in any of the samples of affected tissue from 4 participants with an unrelated cerebrovascular malformation or in any of the samples from the 6 controls. The prevalence of the mutant allele in affected tissues ranged from 1.0 to 18.1%. Extracellular signal-regulated kinase activity was modestly increased during transgenic expression of mutant Gαq.
Conclusions: The Sturge-Weber syndrome and port-wine stains are caused by a somatic activating mutation in GNAQ. This finding confirms a long-standing hypothesis. (Funded by the National Institutes of Health and Hunter's Dream for a Cure Foundation.).
Figures

Comment in
-
[Sturge-Weber syndrome and port-wine stains: causative role of postzygotic somatic mutations in GNAQ].Ann Dermatol Venereol. 2013 Oct;140(10):658-9. doi: 10.1016/j.annder.2013.06.005. Epub 2013 Jul 27. Ann Dermatol Venereol. 2013. PMID: 24090900 French. No abstract available.
References
-
- Comi AM. Update on Sturge-Weber syndrome: diagnosis, treatment, quantitative measures, and controversies. Lymphat Res Biol. 2007;5:257–64. - PubMed
-
- Piram M, Lorette G, Sirinelli D, Herbreteau D, Giraudeau B, Maruani A. Sturge-Weber syndrome in patients with facial port-wine stain. Pediatr Dermatol. 2012;29:32–7. - PubMed
-
- Ch’ng S, Tan ST. Facial port-wine stains — clinical stratification and risks of neuro-ocular involvement. J Plast Reconstr Aesthet Surg. 2008;61:889–93. - PubMed
-
- Greene AK, Taber SF, Ball KL, Padwa BL, Mulliken JB. Sturge-Weber syndrome: soft-tissue and skeletal overgrowth. J Craniofac Surg. 2009;20(Suppl 1):617–21. [Erratum, J Craniofac Surg 2009;20:1629–30.] - PubMed
-
- Gasparini G, Perugini M, Vetrano S, Cassoni A, Fini G. Angiodysplasia with osteohypertrophy affecting the oromaxillofacial area: clinical findings. J Craniofac Surg. 2001;12:485–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
