Architectural remodeling of the tonoplast during fluid-phase endocytosis
- PMID: 23656870
- PMCID: PMC3908939
- DOI: 10.4161/psb.24793
Architectural remodeling of the tonoplast during fluid-phase endocytosis
Abstract
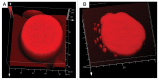
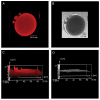
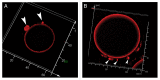
During fluid phase endocytosis (FPE) in plant storage cells, the vacuole receives a considerable amount of membrane and fluid contents. If allowed to accumulate over a period of time, the enlarging tonoplast and increase in fluids would invariably disrupt the structural equilibrium of the mature cells. Therefore, a membrane retrieval process must exist that will guarantee membrane homeostasis in light of tonoplast expansion by membrane addition during FPE. We examined the morphological changes to the vacuolar structure during endocytosis in red beet hypocotyl tissue using scanning laser confocal microscopy and immunohistochemistry. The heavily pigmented storage vacuole allowed us to visualize all architectural transformations during treatment. When red beet tissue was incubated in 200 mM sucrose, a portion of the sucrose accumulated entered the cell by means of FPE. The accumulation process was accompanied by the development of vacuole-derived vesicles which transiently counterbalanced the addition of surplus endocytic membrane during rapid rates of endocytosis. Topographic fluorescent confocal micrographs showed an ensuing reduction in the size of the vacuole-derived vesicles and further suggest their reincorporation into the vacuole to maintain vacuolar unity and solute concentration.
Keywords: exocytosis; retrograde vesicles; sucrose transport; tonoplast.
Figures



References
-
- Baluška F, Baroja-Fernandez E, Pozueta-Romero J, Hlavacka J, Etxeberria E, Samaj J. Endocytic uptake of nutrients, cell wall molecules, and fluidized cell wall portions into heterotrophic plant cells. In: Šamaj J, Baluška F, Menzel D, ed(s) Plant Endocytosis, Plant Cell Monograph, Springer Verlag, Berlin. 2005:1-17.
-
- Etxeberria E, Gonzalez P, Pozueta-Romero J. Sucrose transport into Citrus juice cells: evidence for an endocytic transport system. J Am Soc Hortic Sci. 2005;130:269–74. b.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
