Glutamate receptor-like channel3.3 is involved in mediating glutathione-triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis
- PMID: 23656893
- PMCID: PMC3700673
- DOI: 10.1104/pp.113.217208
Glutamate receptor-like channel3.3 is involved in mediating glutathione-triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis
Abstract
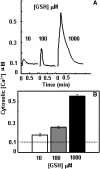
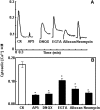
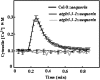
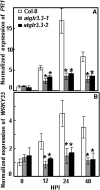
The tripeptide reduced glutathione (GSH; γ-glutamate [Glu]-cysteine [Cys]-glycine) is a major endogenous antioxidant in both animal and plant cells. It also functions as a neurotransmitter mediating communication among neurons in the central nervous system of animals through modulating specific ionotropic Glu receptors (GLRs) in the membrane. Little is known about such signaling roles in plant cells. Here, we report that transient rises in cytosolic calcium triggered by exogenous GSH in Arabidopsis (Arabidopsis thaliana) leaves were sensitive to GLR antagonists and abolished in loss-of-function atglr3.3 mutants. Like the GSH biosynthesis-defective mutant PHYTOALEXIN DEFICIENT2, atglr3.3 showed enhanced susceptibility to the bacterial pathogen Pseudomonas syringae pv tomato DC3000. Pathogen-induced defense marker gene expression was also decreased in atglr3.3 mutants. Twenty-seven percent of genes that were rapidly responsive to GSH treatment of seedlings were defense genes, most of which were dependent on functional AtGLR3.3, while GSH suppressed pathogen propagation through the AtGLR3.3-dependent pathway. Eight previously identified putative AtGLR3.3 ligands, GSH, oxidized glutathione, alanine, asparagine, Cys, Glu, glycine, and serine, all elicited the AtGLR3.3-dependent cytosolic calcium transients, but only GSH and Cys induced the defense response, with the Glu-induced AtGLR3.3-dependent transcription response being much less apparent than that triggered by GSH. Together, these observations suggest that AtGLR3.3 is required for several signaling effects mediated by extracellular GSH, even though these effects may not be causally related.
Figures



Similar articles
-
Involvement of the glutamate receptor AtGLR3.3 in plant defense signaling and resistance to Hyaloperonospora arabidopsidis.Plant J. 2013 Nov;76(3):466-80. doi: 10.1111/tpj.12311. Epub 2013 Sep 19. Plant J. 2013. PMID: 23952652
-
AtGLR3.4, a glutamate receptor channel-like gene is sensitive to touch and cold.Planta. 2005 Oct;222(3):418-27. doi: 10.1007/s00425-005-1551-3. Epub 2005 Apr 28. Planta. 2005. PMID: 15864638
-
Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile.Plant Physiol. 2006 Nov;142(3):963-71. doi: 10.1104/pp.106.088989. Epub 2006 Sep 29. Plant Physiol. 2006. PMID: 17012403 Free PMC article.
-
Merging Signaling with Structure: Functions and Mechanisms of Plant Glutamate Receptor Ion Channels.Annu Rev Plant Biol. 2023 May 22;74:415-452. doi: 10.1146/annurev-arplant-070522-033255. Epub 2023 Feb 28. Annu Rev Plant Biol. 2023. PMID: 36854472 Free PMC article. Review.
-
Structural and Functional Diversity of Glutamate Receptors-Like Channels in Plants.Physiol Plant. 2025 May-Jun;177(3):e70313. doi: 10.1111/ppl.70313. Physiol Plant. 2025. PMID: 40468591 Review.
Cited by
-
Genome-Wide Identification and Expression Analysis of BraGLRs Reveal Their Potential Roles in Abiotic Stress Tolerance and Sexual Reproduction.Cells. 2022 Nov 22;11(23):3729. doi: 10.3390/cells11233729. Cells. 2022. PMID: 36496989 Free PMC article.
-
Delineating Calcium Signaling Machinery in Plants: Tapping the Potential through Functional Genomics.Curr Genomics. 2021 Dec 30;22(6):404-439. doi: 10.2174/1389202922666211130143328. Curr Genomics. 2021. PMID: 35340362 Free PMC article. Review.
-
Damage-Associated Molecular Pattern-Triggered Immunity in Plants.Front Plant Sci. 2019 May 22;10:646. doi: 10.3389/fpls.2019.00646. eCollection 2019. Front Plant Sci. 2019. PMID: 31191574 Free PMC article. Review.
-
Roles of Glutathione in Mediating Abscisic Acid Signaling and Its Regulation of Seed Dormancy and Drought Tolerance.Genes (Basel). 2021 Oct 14;12(10):1620. doi: 10.3390/genes12101620. Genes (Basel). 2021. PMID: 34681014 Free PMC article. Review.
-
Root apex transition zone as oscillatory zone.Front Plant Sci. 2013 Oct 2;4:354. doi: 10.3389/fpls.2013.00354. eCollection 2013. Front Plant Sci. 2013. PMID: 24106493 Free PMC article.
References
-
- Acosta IF, Farmer EE. (2010) Jasmonates. The Arabidopsis Book 8: e0129, doi/10.1199/tab.0129 - DOI - PMC - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Aouini A, Hernould M, Ariizumi T, Matsukura C, Ezura H, Asamizu E. (2012) Overexpression of the tomato glutamate receptor-like genes SlGLR1.1 and SlGLR3.5 hinders Ca2+ utilization and promotes hypersensitivity to Na+ and K+ stresses. Plant Biotechnol 29: 229–235
-
- Aoyama K, Watabe M, Nakaki T. (2008) Regulation of neuronal glutathione synthesis. J Pharmacol Sci 108: 227–238 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

