CD14 contributes to warm hepatic ischemia-reperfusion injury in mice
- PMID: 23656919
- PMCID: PMC3719185
- DOI: 10.1097/SHK.0b013e318299d1a7
CD14 contributes to warm hepatic ischemia-reperfusion injury in mice
Abstract
Introduction: Ischemia/reperfusion (I/R) of the liver contributes to the pathobiology of liver injury in transplantation, liver surgery, and hemorrhagic shock. Ischemia/reperfusion induces an inflammatory response that is driven, in part, by Toll-like receptor 4 (TLR) signaling. CD14 is known to participate in the function of TLR4. We hypothesized that CD14 would be involved in the pathobiology of warm hepatic I/R.
Methods: Using a 70% liver inflow inclusion model, CD14 knockout and wild-type (WT) mice were subjected to 1-h warm ischemia followed by reperfusion. CD14 mRNA, circulating transaminase, interleukin 6, soluble CD14, and high-mobility group box 1 (HMGB1) levels were measured. CD14 neutralizing antibody or isotype control antibody was given before ischemia or reperfusion for CD14 blockade in WT mice. Recombinant HMGB1 was given before reperfusion in some experiments to test if liver injury worsens.
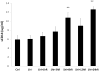
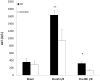
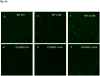
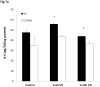
Results: There was an upregulation of CD14 mRNA in reperfused livers together with increased soluble CD14 levels in the circulation. Compared with WT control mice, CD14 knockout mice had much lower alanine aminotransferase and interleukin 6 levels at 6 and 24 h following I/R, and much less liver necrosis by histology. TUNEL (terminal deoxynucleotidyl-transferase dUTP nick end labeling) staining displayed less apoptosis at 24 h in the absence of CD14. CD14 blockage by neutralizing antibody also attenuated liver injury and the inflammatory response in C57BL/6 mice following I/R, but did not provide additional protection to TLR4 mutant C3H/Hej mice. CD14 deficiency did not change circulating HMGB1 levels following I/R (6 h). A dose of recombinant HMGB1, which worsened hepatic injury when given before reperfusion in WT mice, did not increase liver damage in CD14-deficient mice.
Conclusions: CD14 is actively involved in hepatic I/R injury. Its deficiency or blockade ischemia attenuates liver injury and inflammatory response. CD14 mediates liver damage and inflammatory responses in the setting of warm hepatic I/R in mice.
Figures











Similar articles
-
CD14 blockade to prevent ischemic injury to donor organs.Transpl Immunol. 2022 Jun;72:101580. doi: 10.1016/j.trim.2022.101580. Epub 2022 Mar 11. Transpl Immunol. 2022. PMID: 35283329 Free PMC article. Review.
-
The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion.J Exp Med. 2005 Apr 4;201(7):1135-43. doi: 10.1084/jem.20042614. Epub 2005 Mar 28. J Exp Med. 2005. PMID: 15795240 Free PMC article.
-
HMGB1 translocation and expression is caused by warm ischemia reperfusion injury, but not by partial hepatectomy in rats.Exp Mol Pathol. 2011 Oct;91(2):502-8. doi: 10.1016/j.yexmp.2011.05.005. Epub 2011 May 27. Exp Mol Pathol. 2011. PMID: 21640719
-
Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling.Mol Med. 2015 Mar 13;20(1):639-48. doi: 10.2119/molmed.2014.00076. Mol Med. 2015. PMID: 25375408 Free PMC article.
-
Redox therapeutics in hepatic ischemia reperfusion injury.World J Hepatol. 2014 Jan 27;6(1):1-8. doi: 10.4254/wjh.v6.i1.1. World J Hepatol. 2014. PMID: 24653789 Free PMC article. Review.
Cited by
-
Magnesium sulfate inhibits binding of lipopolysaccharide to THP-1 cells by reducing expression of cluster of differentiation 14.Inflammopharmacology. 2019 Apr;27(2):249-260. doi: 10.1007/s10787-019-00568-7. Epub 2019 Feb 5. Inflammopharmacology. 2019. PMID: 30721372
-
Mincle suppresses Toll-like receptor 4 activation.J Leukoc Biol. 2016 Jul;100(1):185-94. doi: 10.1189/jlb.3A0515-185R. Epub 2016 Jan 8. J Leukoc Biol. 2016. PMID: 26747838 Free PMC article.
-
Emerging role of high-mobility group box 1 (HMGB1) in liver diseases.Mol Med. 2013 Nov 8;19(1):357-66. doi: 10.2119/molmed.2013.00099. Mol Med. 2013. PMID: 24306421 Free PMC article. Review.
-
CD14 blockade to prevent ischemic injury to donor organs.Transpl Immunol. 2022 Jun;72:101580. doi: 10.1016/j.trim.2022.101580. Epub 2022 Mar 11. Transpl Immunol. 2022. PMID: 35283329 Free PMC article. Review.
-
New insights into Nod-like receptors (NLRs) in liver diseases.Int J Physiol Pathophysiol Pharmacol. 2018 Mar 10;10(1):1-16. eCollection 2018. Int J Physiol Pathophysiol Pharmacol. 2018. PMID: 29593846 Free PMC article. Review.
References
-
- Clemens MG, Bauer M, Pannen BH, Bauer I, Zhang JX. Remodeling of hepatic microvascular responsiveness after ischemia/reperfusion. Shock. 1997;8 (2):80–85. - PubMed
-
- Wu HS, Zhang JX, Wang L, Tian Y, Wang H, Rotstein O. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3 (2):250–253. - PubMed
-
- Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173 (12):7115–7119. - PubMed
-
- Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, Busuttil RW, Kupiec-Weglinski JW. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13 (10):1435–1443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

