Developmental changes in GABAergic neurotransmission to presympathetic and cardiac parasympathetic neurons in the brainstem
- PMID: 23657280
- PMCID: PMC3742984
- DOI: 10.1152/jn.01054.2012
Developmental changes in GABAergic neurotransmission to presympathetic and cardiac parasympathetic neurons in the brainstem
Abstract
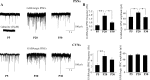
Cardiovascular function is regulated by a dynamic balance composed of sympathetic and parasympathetic activity. Sympathoexcitatory presympathetic neurons (PSNs) in the rostral ventrolateral medulla project directly to cardiac and vasomotor sympathetic preganglionic neurons in the spinal cord. In proximity to the PSNs in the medulla, there are preganglionic cardiac vagal neurons (CVNs) within the nucleus ambiguus, which are critical for parasympathetic control of heart rate. Both CVNs and PSNs receive GABAergic synaptic inputs that change with challenges such as hypoxia and hypercapnia (H/H). Autonomic control of cardiovascular function undergoes significant changes during early postnatal development; however, little is known regarding postnatal maturation of GABAergic neurotransmission to these neurons. In this study, we compared changes in GABAergic inhibitory postsynaptic currents (IPSCs) in CVNs and PSNs under control conditions and during H/H in postnatal day 2-5 (P5), 16-20 (P20), and 27-30 (P30) rats using an in vitro brainstem slice preparation. There was a significant enhancement in GABAergic neurotransmission to both CVNs and PSNs at age P20 compared with P5 and P30, with a more pronounced increase in PSNs. H/H did not significantly alter this enhanced GABAergic neurotransmission to PSNs in P20 animals. However, the frequency of GABAergic IPSCs in PSNs was reduced by H/H in P5 and P30 animals. In CVNs, H/H elicited an inhibition of GABAergic neurotransmission in all ages studied, with the most pronounced inhibition occurring at P20. In conclusion, there are critical development periods at which significant rearrangement occurs in the central regulation of cardiovascular function.
Keywords: brainstem; development; neurons; parasympathetic; presympathetic.
Figures



References
-
- Adolph EF. Ranges of heart rates and their regulations at various ages (rat). Am J Physiol 212: 595–602, 1967 - PubMed
-
- Bouairi E, Neff R, Evans C, Gold A, Andresen MC, Mendelowitz D. Respiratory sinus arrhythmia in freely moving and anesthetized Rats. J Appl Physiol 97: 1431–1436, 2004 - PubMed
-
- Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res 56: 359–369, 1985 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

