Primary motor cortical discharge during force field adaptation reflects muscle-like dynamics
- PMID: 23657285
- PMCID: PMC3742991
- DOI: 10.1152/jn.00109.2012
Primary motor cortical discharge during force field adaptation reflects muscle-like dynamics
Abstract
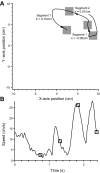
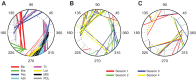
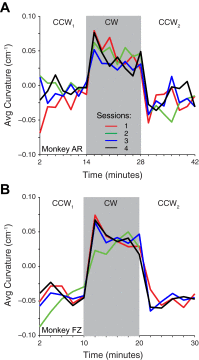
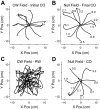
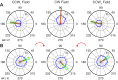
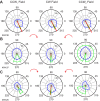
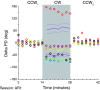
We often make reaching movements having similar trajectories within very different mechanical environments, for example, with and without an added load in the hand. Under these varying conditions, our kinematic intentions must be transformed into muscle commands that move the limbs. Primary motor cortex (M1) has been implicated in the neural mechanism that mediates this adaptation to new movement dynamics, but our recent experiments suggest otherwise. We have recorded from electrode arrays that were chronically implanted in M1 as monkeys made reaching movements under two different dynamic conditions: the movements were opposed by either a clockwise or counterclockwise velocity-dependent force field acting at the hand. Under these conditions, the preferred direction (PD) of neural discharge for nearly all neurons rotated in the direction of the applied field, as did those of proximal limb electromyograms (EMGs), although the median neural rotation was significantly smaller than that of muscles. For a given neuron, the rotation angle was very consistent, even across multiple sessions. Within the limits of measurement uncertainty, both the neural and EMG changes occurred nearly instantaneously, reaching a steady state despite ongoing behavioral adaptation. Our results suggest that M1 is not directly involved in the adaptive changes that occurred within an experimental session. Rather, most M1 neurons are directly related to the dynamics of muscle activation that themselves reflect the external load. It appears as though gain modulation, the differential recruitment of M1 neurons by higher motor areas, can account for the load and behavioral adaptation-related changes in M1 discharge.
Keywords: curl fields; inverse dynamics model; monkey; reaching movements.
Figures





References
-
- Ajemian R, Bullock D, Grossberg S. A model of movement coordinates in the motor cortex: posture-dependent changes in the gain and direction of single cell tuning curves. Cereb Cortex 11: 1124–1135, 2001 - PubMed
-
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol 64: 133–150, 1990 - PubMed
-
- Bhushan N, Shadmehr R. Computational nature of human adaptive control during learning of reaching movements in force fields. Biol Cybern 81: 39–60, 1999 - PubMed
-
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature 382: 252–255, 1996 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

