Cadherin-mediated cell-cell adhesion and signaling in the skeleton
- PMID: 23657489
- PMCID: PMC4272239
- DOI: 10.1007/s00223-013-9733-7
Cadherin-mediated cell-cell adhesion and signaling in the skeleton
Abstract
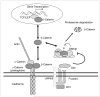
Direct cell-to-cell interactions via cell adhesion molecules, in particular cadherins, are critical for morphogenesis, tissue architecture, and cell sorting and differentiation. Partially overlapping, yet distinct roles of N-cadherin (cadherin-2) and cadherin-11 in the skeletal system have emerged from mouse genetics and in vitro studies. Both cadherins are important for precursor commitment to the osteogenic lineage, and genetic ablation of Cdh2 and Cdh11 results in skeletal growth defects and impaired bone formation. While Cdh11 defines the osteogenic lineage, persistence of Cdh2 in osteoblasts in vivo actually inhibits their terminal differentiation and impairs bone formation. The action of cadherins involves both cell-cell adhesion and interference with intracellular signaling, and in particular the Wnt/β-catenin pathway. Both cadherin-2 and cadherin-11 bind to β-catenin, thus modulating its cytoplasmic pools and transcriptional activity. Recent data demonstrate that cadherin-2 also interferes with Lrp5/6 signaling by sequestering these receptors in inactive pools via axin binding. These data extend the biologic action of cadherins in bone forming cells, and provide novel mechanisms for development of therapeutic strategies aimed at enhancing bone formation.
Figures


References
-
- Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2:81–94. - PubMed
-
- Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. - PubMed
-
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. - PubMed
-
- Marie PJ. Signaling pathways affecting skeletal health. Curr Osteoporos Rep. 10:190–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

