Proteolytic processing of the Yersinia pestis YapG autotransporter by the omptin protease Pla and the contribution of YapG to murine plague pathogenesis
- PMID: 23657527
- PMCID: PMC3749520
- DOI: 10.1099/jmm.0.056275-0
Proteolytic processing of the Yersinia pestis YapG autotransporter by the omptin protease Pla and the contribution of YapG to murine plague pathogenesis
Abstract
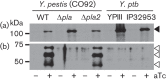
Autotransporter protein secretion represents one of the simplest forms of secretion across Gram-negative bacterial membranes. Once secreted, autotransporter proteins either remain tethered to the bacterial surface or are released following proteolytic cleavage. Autotransporters possess a diverse array of virulence-associated functions such as motility, cytotoxicity, adherence and autoaggregation. To better understand the role of autotransporters in disease, our research focused on the autotransporters of Yersinia pestis, the aetiological agent of plague. Y. pestis strain CO92 has nine functional conventional autotransporters, referred to as Yaps for Yersinia autotransporter proteins. Three Yaps have been directly implicated in virulence using established mouse models of plague infection (YapE, YapJ and YapK). Whilst previous studies from our laboratory have shown that most of the CO92 Yaps are cell associated, YapE and YapG are processed and released by the omptin protease Pla. In this study, we identified the Pla cleavage sites in YapG that result in many released forms of YapG in Y. pestis, but not in the evolutionarily related gastrointestinal pathogen, Yersinia pseudotuberculosis, which lacks Pla. Furthermore, we showed that YapG does not contribute to Y. pestis virulence in established mouse models of bubonic and pneumonic infection. As Y. pestis has a complex life cycle involving a wide range of mammalian hosts and a flea vector for transmission, it remains to be elucidated whether YapG has a measurable role in any other stage of plague disease.
Figures

Similar articles
-
Acquisition of omptin reveals cryptic virulence function of autotransporter YapE in Yersinia pestis.Mol Microbiol. 2013 Jul;89(2):276-87. doi: 10.1111/mmi.12273. Epub 2013 Jun 10. Mol Microbiol. 2013. PMID: 23701256 Free PMC article.
-
Evolution and virulence contributions of the autotransporter proteins YapJ and YapK of Yersinia pestis CO92 and their homologs in Y. pseudotuberculosis IP32953.Infect Immun. 2012 Oct;80(10):3693-705. doi: 10.1128/IAI.00529-12. Epub 2012 Jul 16. Infect Immun. 2012. PMID: 22802344 Free PMC article.
-
Proteolysis of plasminogen activator inhibitor-1 by Yersinia pestis remodulates the host environment to promote virulence.J Thromb Haemost. 2016 Sep;14(9):1833-43. doi: 10.1111/jth.13408. Epub 2016 Aug 19. J Thromb Haemost. 2016. PMID: 27377187 Free PMC article.
-
Using every trick in the book: the Pla surface protease of Yersinia pestis.Adv Exp Med Biol. 2007;603:268-78. doi: 10.1007/978-0-387-72124-8_24. Adv Exp Med Biol. 2007. PMID: 17966423 Review.
-
Fibrinolytic and coagulative activities of Yersinia pestis.Front Cell Infect Microbiol. 2013 Jul 26;3:35. doi: 10.3389/fcimb.2013.00035. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23898467 Free PMC article. Review.
Cited by
-
Environmental Regulation of Yersinia Pathophysiology.Front Cell Infect Microbiol. 2016 Mar 2;6:25. doi: 10.3389/fcimb.2016.00025. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26973818 Free PMC article. Review.
-
Inhibition of outer membrane proteases of the omptin family by aprotinin.Infect Immun. 2015 Jun;83(6):2300-11. doi: 10.1128/IAI.00136-15. Epub 2015 Mar 30. Infect Immun. 2015. PMID: 25824836 Free PMC article.
-
Adhesive properties of YapV and paralogous autotransporter proteins of Yersinia pestis.Infect Immun. 2015 May;83(5):1809-19. doi: 10.1128/IAI.00094-15. Epub 2015 Feb 17. Infect Immun. 2015. PMID: 25690102 Free PMC article.
-
Yersinia pestis: the Natural History of Plague.Clin Microbiol Rev. 2020 Dec 9;34(1):e00044-19. doi: 10.1128/CMR.00044-19. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33298527 Free PMC article. Review.
-
Two Isoforms of Yersinia pestis Plasminogen Activator Pla: Intraspecies Distribution, Intrinsic Disorder Propensity, and Contribution to Virulence.PLoS One. 2016 Dec 9;11(12):e0168089. doi: 10.1371/journal.pone.0168089. eCollection 2016. PLoS One. 2016. PMID: 27936190 Free PMC article.
References
-
- Allsopp L. P., Totsika M., Tree J. J., Ulett G. C., Mabbett A. N., Wells T. J., Kobe B., Beatson S. A., Schembri M. A. (2010). UpaH is a newly identified autotransporter protein that contributes to biofilm formation and bladder colonization by uropathogenic Escherichia coli CFT073. Infect Immun 78, 1659–1669 10.1128/IAI.01010-09 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

