Chemoattractant stimulation of TORC2 is regulated by receptor/G protein-targeted inhibitory mechanisms that function upstream and independently of an essential GEF/Ras activation pathway in Dictyostelium
- PMID: 23657816
- PMCID: PMC3694798
- DOI: 10.1091/mbc.E13-03-0130
Chemoattractant stimulation of TORC2 is regulated by receptor/G protein-targeted inhibitory mechanisms that function upstream and independently of an essential GEF/Ras activation pathway in Dictyostelium
Abstract
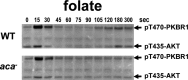
Global stimulation of Dictyostelium with different chemoattractants elicits multiple transient signaling responses, including synthesis of cAMP and cGMP, actin polymerization, activation of kinases ERK2, TORC2, and phosphatidylinositide 3-kinase, and Ras-GTP accumulation. Mechanisms that down-regulate these responses are poorly understood. Here we examine transient activation of TORC2 in response to chemically distinct chemoattractants, cAMP and folate, and suggest that TORC2 is regulated by adaptive, desensitizing responses to stimulatory ligands that are independent of downstream, feedback, or feedforward circuits. Cells with acquired insensitivity to either folate or cAMP remain fully responsive to TORC2 activation if stimulated with the other ligand. Thus TORC2 responses to cAMP or folate are not cross-inhibitory. Using a series of signaling mutants, we show that folate and cAMP activate TORC2 through an identical GEF/Ras pathway but separate receptors and G protein couplings. Because the common GEF/Ras pathway also remains fully responsive to one chemoattractant after desensitization to the other, GEF/Ras must act downstream and independent of adaptation to persistent ligand stimulation. When initial chemoattractant concentrations are immediately diluted, cells rapidly regain full responsiveness. We suggest that ligand adaptation functions in upstream inhibitory pathways that involve chemoattractant-specific receptor/G protein complexes and regulate multiple response pathways.
Figures







References
-
- Chakrabarti S, Regec A, Gintzler AR. Chronic morphine acts via a protein kinase C(gamma)-G(beta)-adenylyl cyclase complex to augment phosphorylation of G(beta) and G(betagamma) stimulatory adenylyl cyclase signaling. Brain Res Mol Brain Res. 2005;138:94–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

