Bilateral changes of cannabinoid receptor type 2 protein and mRNA in the dorsal root ganglia of a rat neuropathic pain model
- PMID: 23657829
- PMCID: PMC3707359
- DOI: 10.1369/0022155413491269
Bilateral changes of cannabinoid receptor type 2 protein and mRNA in the dorsal root ganglia of a rat neuropathic pain model
Abstract
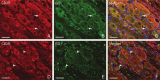
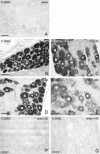
Cannabinoid receptor type 2 (CB2R) plays a critical role in nociception. In contrast to cannabinoid receptor type 1 ligands, CB2R agonists do not produce undesirable central nervous system effects and thus promise to treat neuropathic pain that is often resistant to medical therapy. In the study presented here, we evaluated the bilateral distribution of the CB2R protein and messenger RNA (mRNA) in rat dorsal root ganglia (DRG) after unilateral peripheral nerve injury using immunohistochemistry, western blot, and in situ hybridization analysis. Unilateral chronic constriction injury (CCI) of the sciatic nerve induced neuropathic pain behavior and bilateral elevation of both CB2R protein and mRNA in lumbar L4-L5 as well as cervical C7-C8 DRG when compared with naive animals. CB2R protein and mRNA were increased not only in DRG neurons but also in satellite glial cells. The fact that changes appear bilaterally and (albeit at a lower level) even in the remote cervical DRG can be related to propagation of neuroinflammation alongside the neuraxis and to the neuroprotective effects of CB2R.
Keywords: remote neuroinflammation; satellite glial cells; unilateral nerve injury.
Conflict of interest statement
Figures









References
-
- Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, Birch R, Benham C, Bountra C, Chessell IP, et al. 2008. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 138:667–680 - PubMed
-
- Austin PJ, Moalem-Taylor G. 2010. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 229:26–50 - PubMed
-
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. 2006. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. J Neurosci. 23:1530–1538 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

