Control of RhoA methylation by carboxylesterase I
- PMID: 23658012
- PMCID: PMC3696689
- DOI: 10.1074/jbc.M113.467407
Control of RhoA methylation by carboxylesterase I
Abstract
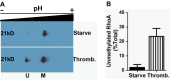
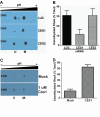
A number of proteins that play key roles in cell signaling are post-translationally modified by the prenylation pathway. The final step in this pathway is methylation of the carboxyl terminus of the prenylated protein by isoprenylcysteine carboxylmethyltransferase. Due to the impact of methylation on Rho function, we sought to determine if the process was reversible and hence could control Rho function in a dynamic fashion. Elevating isoprenylcysteine carboxylmethyltransferase activity in cells has profound effects on MDA-MB-231 cell morphology, implying the presence of a pool of unmethylated prenyl proteins in these cells under normal conditions. Using a knockdown approach, we identified a specific esterase, carboxylesterase 1, whose function had a clear impact not only on the methylation status of RhoA but also RhoA activation and cell morphology. These data provide compelling evidence that C-terminal modification of prenyl proteins, rather than being purely a constitutive process, can serve as a point of regulation of function for this important class of protein.
Keywords: Carboxylesterase; Carboxylesterase 1; Isoprenylcysteine Carboxylmethyltransferase; Methylation; Post-translational Modification; Prenylation; Protein Isoprenylation; Protein Methylation; Rhoa; rhoA.
Figures



References
-
- Glomset J. A., Farnsworth C. C. (1994) Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu. Rev. Cell Biol. 10, 181–205 - PubMed
-
- Zhang F. L., Casey P. J. (1996) Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65, 241–269 - PubMed
-
- Casey P. J., Seabra M. C. (1996) Protein prenyltransferases. J. Biol. Chem. 271, 5289–5292 - PubMed
-
- Ashby M. N. (1998) CaaX converting enzymes. Curr. Opin. Lipidol. 9, 99–102 - PubMed
-
- Young S. G., Ambroziak P., Kim E., Clarke S. (2001) Postisoprenylation protein processing: CXXX (CaaX) endoproteases and isoprenylcysteine carboxyl methyltransferase. The Enzymes (Tamanoi F., Sigman D. S., eds) 3rd Ed., vol. 21, pp. 156–213, Academic Press, San Diego, CA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

