Structural basis for complement evasion by Lyme disease pathogen Borrelia burgdorferi
- PMID: 23658013
- PMCID: PMC3696643
- DOI: 10.1074/jbc.M113.459040
Structural basis for complement evasion by Lyme disease pathogen Borrelia burgdorferi
Abstract
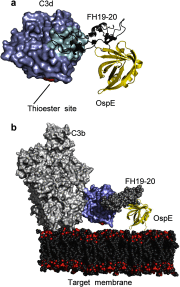
Borrelia burgdorferi spirochetes that cause Lyme borreliosis survive for a long time in human serum because they successfully evade the complement system, an important arm of innate immunity. The outer surface protein E (OspE) of B. burgdorferi is needed for this because it recruits complement regulator factor H (FH) onto the bacterial surface to evade complement-mediated cell lysis. To understand this process at the molecular level, we used a structural approach. First, we solved the solution structure of OspE by NMR, revealing a fold that has not been seen before in proteins involved in complement regulation. Next, we solved the x-ray structure of the complex between OspE and the FH C-terminal domains 19 and 20 (FH19-20) at 2.83 Å resolution. The structure shows that OspE binds FH19-20 in a way similar to, but not identical with, that used by endothelial cells to bind FH via glycosaminoglycans. The observed interaction of OspE with FH19-20 allows the full function of FH in down-regulation of complement activation on the bacteria. This reveals the molecular basis for how B. burgdorferi evades innate immunity and suggests how OspE could be used as a potential vaccine antigen.
Keywords: Bb-CRASP; Borrelia; Complement; Immune Evasion; Microbial Pathogenesis; NMR; Outer Surface Protein E; Protein Complex Structure; Protein Structure; X-ray Crystallography.
Figures
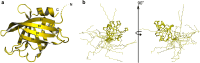


References
-
- Barbour A. G., Maupin G. O., Teltow G. J., Carter C. J., Piesman J. (1996) Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum. Possible agent of a Lyme disease-like illness. J. Infect. Dis. 173, 403–409 - PubMed
-
- Centers for Disease Control and Prevention (CDC) (1996) Lyme disease. United States, 1995. MMWR Morb. Mortal. Wkly. Rep. 45, 481–484 - PubMed
-
- Barbour A. G., Fish D. (1993) The biological and social phenomenon of Lyme disease. Science 260, 1610–1616 - PubMed
-
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. (1983) The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308, 733–740 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

