Thalamic control of neocortical area formation in mice
- PMID: 23658181
- PMCID: PMC3732791
- DOI: 10.1523/JNEUROSCI.5786-12.2013
Thalamic control of neocortical area formation in mice
Abstract
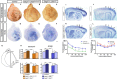
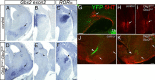
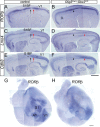
The mammalian neocortex undergoes dramatic transformation during development, from a seemingly homogenous sheet of neuroepithelial cells into a complex structure that is tangentially divided into discrete areas. This process is thought to be controlled by a combination of intrinsic patterning mechanisms within the cortex and afferent axonal projections from the thalamus. However, roles of thalamic afferents in the formation of areas are still poorly understood. In this study, we show that genetically increasing or decreasing the size of the lateral geniculate nucleus of the mouse thalamus resulted in a corresponding change in the size of the primary visual area. Furthermore, elimination of most thalamocortical projections from the outset of their development resulted in altered areal gene expression patterns, particularly in the primary visual and somatosensory areas, where they lost sharp boundaries with adjacent areas. Together, these results demonstrate the critical roles of thalamic afferents in the establishment of neocortical areas.
Figures
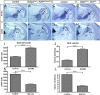
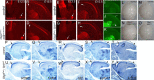
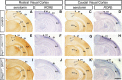
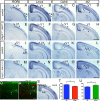



Comment in
-
Thalamic afferents and neocortical arealization: an ongoing journey.J Neurosci. 2013 Aug 28;33(35):13938-9. doi: 10.1523/JNEUROSCI.2859-13.2013. J Neurosci. 2013. PMID: 23986230 Free PMC article. No abstract available.
References
-
- Bulfone A, Puelles L, Porteus MH, Frohman MA, Martin GR, Rubenstein JL. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J Neurosci. 1993;13:3155–3172. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
