PET imaging of chemokine receptors in vascular injury-accelerated atherosclerosis
- PMID: 23658218
- PMCID: PMC4251467
- DOI: 10.2967/jnumed.112.114777
PET imaging of chemokine receptors in vascular injury-accelerated atherosclerosis
Abstract
Atherosclerosis is the pathophysiologic process behind lethal cardiovascular diseases. It is a chronic inflammatory progression. Chemokines can strongly affect the initiation and progression of atherosclerosis by controlling the trafficking of inflammatory cells in vivo through interaction with their receptors. Some chemokine receptors have been reported to play an important role in plaque development and stability. However, the diagnostic potential of chemokine receptors has not yet been explored. The purpose of this study was to develop a positron emitter-radiolabeled probe to image the upregulation of chemokine receptor in a wire-injury-accelerated apolipoprotein E knockout (ApoE(-/-)) mouse model of atherosclerosis.
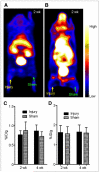
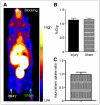
Methods: A viral macrophage inflammatory protein II (vMIP-II) was used to image the upregulation of multiple chemokine receptors through conjugation with DOTA for (64)Cu radiolabeling and PET. Imaging studies were performed at 2 and 4 wk after injury in both wire-injured ApoE(-/-) and wild-type C57BL/6 mice. Competitive PET blocking studies with nonradiolabeled vMIP-II were performed to confirm the imaging specificity. Specific PET blocking with individual chemokine receptor antagonists was also performed to verify the upregulation of a particular chemokine receptor. In contrast, (18)F-FDG PET imaging was performed in both models to evaluate tracer uptake. Immunohistochemistry on the injury and sham tissues was performed to assess the upregulation of chemokine receptors.
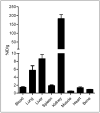
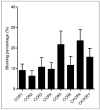
Results: (15)O-CO PET showed decreased blood volume in the femoral artery after the injury. (64)Cu-DOTA-vMIP-II exhibited fast in vivo pharmacokinetics with major renal clearance. PET images showed specific accumulation around the injury site, with consistent expression during the study period. Quantitative analysis of tracer uptake at the injury lesion in the ApoE(-/-) model showed a 3-fold increase over the sham-operated site and the sites in the injured wild-type mouse. (18)F-FDG PET showed significantly less tracer accumulation than (64)Cu-DOTA-vMIP-II, with no difference observed between injury and sham sites. PET blocking studies identified chemokine receptor-mediated (64)Cu-DOTA-vMIP-II uptake and verified the presence of 8 chemokine receptors, and this finding was confirmed by immunohistochemistry.
Conclusion: (64)Cu-DOTA-vMIP-II was proven a sensitive and useful PET imaging probe for the detection of 8 up-regulated chemokine receptors in a model of injury-accelerated atherosclerosis.
Keywords: atherosclerosis; chemokine receptors; molecular imaging; positron emission tomography; virus macrophage inflammatory protein.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
