Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies
- PMID: 23658244
- PMCID: PMC3864887
- DOI: 10.1126/scitranslmed.3005365
Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies
Abstract
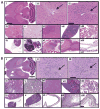
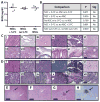
High-grade gliomas are extremely difficult to treat because they are invasive and therefore not curable by surgical resection; the toxicity of current chemo- and radiation therapies limits the doses that can be used. Neural stem cells (NSCs) have inherent tumor-tropic properties that enable their use as delivery vehicles to target enzyme/prodrug therapy selectively to tumors. We used a cytosine deaminase (CD)-expressing clonal human NSC line, HB1.F3.CD, to home to gliomas in mice and locally convert the prodrug 5-fluorocytosine to the active chemotherapeutic 5-fluorouracil. In vitro studies confirmed that the NSCs have normal karyotype, tumor tropism, and CD expression, and are genetically and functionally stable. In vivo biodistribution studies demonstrated NSC retention of tumor tropism, even in mice pretreated with radiation or dexamethasone to mimic clinically relevant adjuvant therapies. We evaluated safety and toxicity after intracerebral administration of the NSCs in non-tumor-bearing and orthotopic glioma-bearing immunocompetent and immunodeficient mice. We detected no difference in toxicity associated with conversion of 5-fluorocytosine to 5-fluorouracil, no NSCs outside the brain, and no histological evidence of pathology or tumorigenesis attributable to the NSCs. The average tumor volume in mice that received HB1.F3.CD NSCs and 5-fluorocytosine was about one-third that of the average volume in control mice. On the basis of these results, we conclude that combination therapy with HB1.F3.CD NSCs and 5-fluorocytosine is safe, nontoxic, and effective in mice. These data have led to approval of a first-in-human study of an allogeneic NSC-mediated enzyme/prodrug-targeted cancer therapy in patients with recurrent high-grade glioma.
Conflict of interest statement
Figures




References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12:495–508. - PubMed
-
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

