A novel bivalent vaccine based on a PB2-knockout influenza virus protects mice from pandemic H1N1 and highly pathogenic H5N1 virus challenges
- PMID: 23658445
- PMCID: PMC3700221
- DOI: 10.1128/JVI.00076-13
A novel bivalent vaccine based on a PB2-knockout influenza virus protects mice from pandemic H1N1 and highly pathogenic H5N1 virus challenges
Abstract
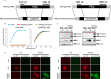
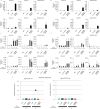
Vaccination is an effective means to protect against influenza virus. Although inactivated and live-attenuated vaccines are currently available, each vaccine has disadvantages (e.g., immunogenicity and safety issues). To overcome these problems, we previously developed a replication-incompetent PB2-knockout (PB2-KO) influenza virus that replicates only in PB2 protein-expressing cells. Here, we generated two PB2-KO viruses whose PB2-coding regions were replaced with the HA genes of either A/California/04/2009 (H1N1pdm09) or A/Vietnam/1203/2004 (H5N1). The resultant viruses comparably, or in some cases more efficiently, induced virus-specific antibodies in the serum, nasal wash, and bronchoalveolar lavage fluid of mice relative to a conventional formalin-inactivated vaccine. Furthermore, mice immunized with these PB2-KO viruses were protected from lethal challenges with not only the backbone virus strain but also strains from which their foreign HAs originated, indicating that PB2-KO viruses with antigenically different HAs could serve as bivalent influenza vaccines.
Figures

References
-
- van der Wouden JC, Bueving HJ, Poole P. 2005. Preventing influenza: an overview of systematic reviews. Respir. Med. 99:1341–1349 - PubMed
-
- Nabel GJ, Fauci AS. 2010. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat. Med. 16:1389–1391 - PubMed
-
- Lambert LC, Fauci AS. 2010. Influenza vaccines for the future. N. Engl. J. Med. 363:2036–2044 - PubMed
-
- Girard MP, Katz J, Pervikov Y, Palkonyay L, Kieny MP. 2010. Report of the 6th meeting on the evaluation of pandemic influenza vaccines in clinical trials World Health Organization, Geneva, Switzerland, 17–18 February 2010. Vaccine 28:6811–6820 - PubMed
-
- Cox RJ, Brokstad KA, Ogra P. 2004. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 59:1–15 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

