Results of switching to milnacipran in fibromyalgia patients with an inadequate response to duloxetine: a phase IV pilot study
- PMID: 23658494
- PMCID: PMC3643187
- DOI: 10.2147/JPR.S43395
Results of switching to milnacipran in fibromyalgia patients with an inadequate response to duloxetine: a phase IV pilot study
Abstract
Background: The purpose of this study was to evaluate the safety, tolerability, and efficacy of milnacipran following a direct switch from duloxetine in fibromyalgia patients experiencing inadequate clinical response to duloxetine after receiving treatment for 6 weeks or longer.
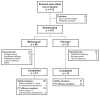
Methods: This exploratory study included 107 patients with fibromyalgia who had been treated with duloxetine 60 mg/day for at least 4 weeks prior to enrollment. Following a 2-week open-label period on duloxetine, patients who had visual analog scale pain scores ≥ 40 and were dissatisfied with current treatment were randomized 4:1 to milnacipran 100 mg/day (n = 86) or placebo (n = 21) for 10 weeks of double-blind treatment. The small placebo group was included solely to blind the study and minimize expectation bias among patients and investigators, and there was no preplanned statistical comparison between treatment groups. The primary efficacy parameter was the percentage of patients rating themselves as "much improved" or "very much improved" on the Patient Global Impression of Change (PGIC) at the final visit. Other efficacy parameters included changes in one-week recall visual analog scale pain, Fibromyalgia Impact Questionnaire Revised (FIQR), and Multiple Ability Self-Report Questionnaire (MASQ).
Results: Of patients switched to milnacipran, 32.9% were classified as PGIC responders, and they also demonstrated improvement in visual analog scale pain, FIQR total, and MASQ total scores (mean changes from baseline were -12.3, -7.77, and -2.39, respectively). Nausea and dizziness were the most common treatment-emergent adverse events in patients switched to milnacipran, reported in 21% and 15%, respectively, of patients in this group.
Conclusion: Results from this exploratory study suggest that switching from duloxetine to milnacipran may be beneficial in some patients with fibromyalgia who have an inadequate response to duloxetine. Further research investigating the efficacy and safety of switching fibromyalgia therapies is warranted.
Keywords: duloxetine; fibromyalgia; milnacipran; switch.
Figures

References
-
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. - PubMed
-
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–1122. - PubMed
-
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. - PubMed
-
- Bennett RM. Clinical manifestations and diagnosis of fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):215–232. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

