A nucleotide sugar transporter involved in glycosylation of the Toxoplasma tissue cyst wall is required for efficient persistence of bradyzoites
- PMID: 23658519
- PMCID: PMC3642066
- DOI: 10.1371/journal.ppat.1003331
A nucleotide sugar transporter involved in glycosylation of the Toxoplasma tissue cyst wall is required for efficient persistence of bradyzoites
Abstract
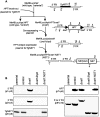
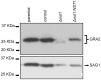
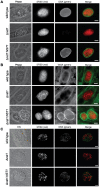
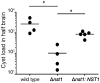
Toxoplasma gondii is an intracellular parasite that transitions from acute infection to a chronic infective state in its intermediate host via encystation, which enables the parasite to evade immune detection and clearance. It is widely accepted that the tissue cyst perimeter is highly and specifically decorated with glycan modifications; however, the role of these modifications in the establishment and persistence of chronic infection has not been investigated. Here we identify and biochemically and biologically characterize a Toxoplasma nucleotide-sugar transporter (TgNST1) that is required for cyst wall glycosylation. Toxoplasma strains deleted for the TgNST1 gene (Δnst1) form cyst-like structures in vitro but no longer interact with lectins, suggesting that Δnst1 strains are deficient in the transport and use of sugars for the biosynthesis of cyst-wall structures. In vivo infection experiments demonstrate that the lack of TgNST1 activity does not detectably impact the acute (tachyzoite) stages of an infection or tropism of the parasite for the brain but that Δnst1 parasites are severely defective in persistence during the chronic stages of the infection. These results demonstrate for the first time the critical role of parasite glycoconjugates in the persistence of Toxoplasma tissue cysts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
A Family of Toxoplasma gondii Genes Related to GRA12 Regulate Cyst Burdens and Cyst Reactivation.mSphere. 2021 Apr 21;6(2):e00182-21. doi: 10.1128/mSphere.00182-21. mSphere. 2021. PMID: 33883265 Free PMC article.
-
Role of Toxoplasma gondii Chloroquine Resistance Transporter in Bradyzoite Viability and Digestive Vacuole Maintenance.mBio. 2019 Aug 6;10(4):e01324-19. doi: 10.1128/mBio.01324-19. mBio. 2019. PMID: 31387907 Free PMC article.
-
Making Home Sweet and Sturdy: Toxoplasma gondii ppGalNAc-Ts Glycosylate in Hierarchical Order and Confer Cyst Wall Rigidity.mBio. 2017 Jan 10;8(1):e02048-16. doi: 10.1128/mBio.02048-16. mBio. 2017. PMID: 28074022 Free PMC article.
-
Protein O- and C-Glycosylation pathways in Toxoplasma gondii and Plasmodium falciparum.Parasitology. 2019 Dec;146(14):1755-1766. doi: 10.1017/S0031182019000040. Epub 2019 Feb 18. Parasitology. 2019. PMID: 30773146 Free PMC article. Review.
-
Developmentally regulated biosynthesis of carbohydrate and storage polysaccharide during differentiation and tissue cyst formation in Toxoplasma gondii.Biochimie. 2003 Mar-Apr;85(3-4):353-61. doi: 10.1016/s0300-9084(03)00076-2. Biochimie. 2003. PMID: 12770773 Review.
Cited by
-
Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response.PLoS Biol. 2014 Apr 29;12(4):e1001845. doi: 10.1371/journal.pbio.1001845. eCollection 2014 Apr. PLoS Biol. 2014. PMID: 24781109 Free PMC article.
-
In vitro Measurement of CMP-Sialic Acid Transporter Activity in Reconstituted Proteoliposomes.Bio Protoc. 2020 Mar 20;10(6):e3551. doi: 10.21769/BioProtoc.3551. eCollection 2020 Mar 20. Bio Protoc. 2020. PMID: 33659525 Free PMC article.
-
Infection by Toxoplasma gondii specifically induces host c-Myc and the genes this pivotal transcription factor regulates.Eukaryot Cell. 2014 Apr;13(4):483-93. doi: 10.1128/EC.00316-13. Epub 2014 Feb 14. Eukaryot Cell. 2014. PMID: 24532536 Free PMC article.
-
Strategies Developed by Toxoplasma gondii to Survive in the Host.Front Microbiol. 2019 Apr 26;10:899. doi: 10.3389/fmicb.2019.00899. eCollection 2019. Front Microbiol. 2019. PMID: 31080445 Free PMC article. Review.
-
GRA25 is a novel virulence factor of Toxoplasma gondii and influences the host immune response.Infect Immun. 2014 Jun;82(6):2595-605. doi: 10.1128/IAI.01339-13. Epub 2014 Apr 7. Infect Immun. 2014. PMID: 24711568 Free PMC article.
References
-
- Dubey JP (1998) Advances in the life cycle of Toxoplasma gondii. Int J Parasitol 28: 1019–1024. - PubMed
-
- Boothroyd JC (2009) Expansion of host range as a driving force in the evolution of Toxoplasma. Mem Inst Oswaldo Cruz 104: 179–184. - PubMed
-
- Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS (1984) Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA 252: 913–917. - PubMed
-
- Luft BJ, Remington JS (1992) Toxoplasmic encephalitis in AIDS. Clin Infect Dis 15: 211–222. - PubMed
-
- Ferguson DJ (2004) Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int J Parasitol 34: 347–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

