Wnt signaling regulates the lineage differentiation potential of mouse embryonic stem cells through Tcf3 down-regulation
- PMID: 23658527
- PMCID: PMC3642041
- DOI: 10.1371/journal.pgen.1003424
Wnt signaling regulates the lineage differentiation potential of mouse embryonic stem cells through Tcf3 down-regulation
Abstract
Canonical Wnt signaling plays a rate-limiting role in regulating self-renewal and differentiation in mouse embryonic stem cells (ESCs). We have previously shown that mutation in the Apc (adenomatous polyposis coli) tumor suppressor gene constitutively activates Wnt signaling in ESCs and inhibits their capacity to differentiate towards ecto-, meso-, and endodermal lineages. However, the underlying molecular and cellular mechanisms through which Wnt regulates lineage differentiation in mouse ESCs remain to date largely unknown. To this aim, we have derived and studied the gene expression profiles of several Apc-mutant ESC lines encoding for different levels of Wnt signaling activation. We found that down-regulation of Tcf3, a member of the Tcf/Lef family and a key player in the control of self-renewal and pluripotency, represents a specific and primary response to Wnt activation in ESCs. Accordingly, rescuing Tcf3 expression partially restored the neural defects observed in Apc-mutant ESCs, suggesting that Tcf3 down-regulation is a necessary step towards Wnt-mediated suppression of neural differentiation. We found that Tcf3 down-regulation in the context of constitutively active Wnt signaling does not result from promoter DNA methylation but is likely to be caused by a plethora of mechanisms at both the RNA and protein level as shown by the observed decrease in activating histone marks (H3K4me3 and H3-acetylation) and the upregulation of miR-211, a novel Wnt-regulated microRNA that targets Tcf3 and attenuates early neural differentiation in mouse ESCs. Our data show for the first time that Wnt signaling down-regulates Tcf3 expression, possibly at both the transcriptional and post-transcriptional levels, and thus highlight a novel mechanism through which Wnt signaling inhibits neuro-ectodermal lineage differentiation in mouse embryonic stem cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

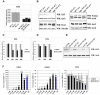



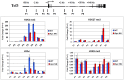

References
-
- Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, et al. (2002) Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet 32: 594–605. - PubMed
-
- Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H (2006) Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun 343: 159–166. - PubMed
-
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 10: 55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

