The pH and pCO2 dependence of sulfate reduction in shallow-sea hydrothermal CO2 - venting sediments (Milos Island, Greece)
- PMID: 23658555
- PMCID: PMC3647119
- DOI: 10.3389/fmicb.2013.00111
The pH and pCO2 dependence of sulfate reduction in shallow-sea hydrothermal CO2 - venting sediments (Milos Island, Greece)
Abstract
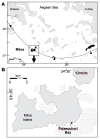
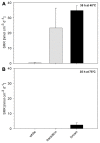
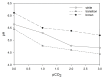
Microbial sulfate reduction (SR) is a dominant process of organic matter mineralization in sulfate-rich anoxic environments at neutral pH. Recent studies have demonstrated SR in low pH environments, but investigations on the microbial activity at variable pH and CO2 partial pressure are still lacking. In this study, the effect of pH and pCO2 on microbial activity was investigated by incubation experiments with radioactive (35)S targeting SR in sediments from the shallow-sea hydrothermal vent system of Milos, Greece, where pH is naturally decreased by CO2 release. Sediments differed in their physicochemical characteristics with distance from the main site of fluid discharge. Adjacent to the vent site (T ~40-75°C, pH ~5), maximal sulfate reduction rates (SRR) were observed between pH 5 and 6. SR in hydrothermally influenced sediments decreased at neutral pH. Sediments unaffected by hydrothermal venting (T ~26°C, pH ~8) expressed the highest SRR between pH 6 and 7. Further experiments investigating the effect of pCO2 on SR revealed a steep decrease in activity when the partial pressure increased from 2 to 3 bar. Findings suggest that sulfate reducing microbial communities associated with hydrothermal vent system are adapted to low pH and high CO2, while communities at control sites required a higher pH for optimal activity.
Keywords: extreme environment; microbial activity; pCO2 effect; pH effect; shallow-seahydrothermal vents; sulfate reduction; sulfate reduction rate.
Figures



References
-
- Amend J., Rogers K., Meyer-Dombard D. (2004). Microbially mediated sulfur -redox: energetics in marine hydrothermal vent systems. Geol. Soc. Am. 379 17–34
-
- Baker-Austin C., Dopson M. (2007). Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 15 165–171 - PubMed
-
- Barton L. L., Fauque G. D. (2009). Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv. Appl. Microbiol. 68 41–98 - PubMed
-
- Bruun A. M., Finster K., Gunnlaugsson H. P., Nürnberg P., Friedrich M. W. (2010). A comprehensive investigation on iron cycling in a freshwater seep including microscopy, cultivation and molecular community analysis. Geomicrobiol. J. 27 15–34
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

