Curcumin inhibits CD4(+) T cell activation, but augments CD69 expression and TGF-β1-mediated generation of regulatory T cells at late phase
- PMID: 23658623
- PMCID: PMC3637266
- DOI: 10.1371/journal.pone.0062300
Curcumin inhibits CD4(+) T cell activation, but augments CD69 expression and TGF-β1-mediated generation of regulatory T cells at late phase
Erratum in
- PLoS One. 2013;8(5). doi: 10.1371/annotation/631b0f02-bf10-4bac-88a3-c986f2b73284
Abstract
Background: Curcumin is a promising candidate for a natural medicinal agent to treat chronic inflammatory diseases. Although CD4(+) T cells have been implicated in the pathogenesis of chronic inflammation, whether curcumin directly regulates CD4(+) T cells has not been definitively established. Here, we showed curcumin-mediated regulation of CD2/CD3/CD28-initiated CD4(+) T cell activation in vitro.
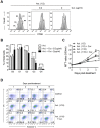
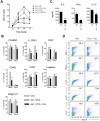
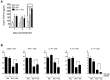
Methodology/principal findings: Primary human CD4(+) T cells were stimulated with anti-CD2/CD3/CD28 antibody-coated beads as an in vitro surrogate system for antigen presenting cell-T cell interaction and treated with curcumin. We found that curcumin suppresses CD2/CD3/CD28-initiated CD4(+) T cell activation by inhibiting cell proliferation, differentiation and cytokine production. On the other hand, curcumin attenuated the spontaneous decline of CD69 expression and indirectly increased expression of CCR7, L-selectin and Transforming growth factor-β1 (TGF-β1) at the late phase of CD2/CD3/CD28-initiated T cell activation. Curcumin-mediated up-regulation of CD69 at late phase was associated with ERK1/2 signaling. Furthermore, TGF-β1 was involved in curcumin-mediated regulation of T cell activation and late-phase generation of regulatory T cells.
Conclusions/significance: Curcumin not merely blocks, but regulates CD2/CD3/CD28-initiated CD4(+) T cell activation by augmenting CD69, CCR7, L-selectin and TGF-β1 expression followed by regulatory T cell generation. These results suggest that curcumin could directly reduce T cell-dependent inflammatory stress by modulating CD4(+) T cell activation at multiple levels.
Conflict of interest statement
Figures



Similar articles
-
Transforming growth factor-beta inhibits human antigen-specific CD4+ T cell proliferation without modulating the cytokine response.Int Immunol. 2003 Dec;15(12):1495-504. doi: 10.1093/intimm/dxg147. Int Immunol. 2003. PMID: 14645158
-
CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production.Cell Death Dis. 2018 Sep 5;9(9):905. doi: 10.1038/s41419-018-0927-9. Cell Death Dis. 2018. PMID: 30185773 Free PMC article.
-
The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development.J Exp Med. 2013 Apr 8;210(4):675-81. doi: 10.1084/jem.20122070. Epub 2013 Mar 25. J Exp Med. 2013. PMID: 23530124 Free PMC article.
-
CD69: from activation marker to metabolic gatekeeper.Eur J Immunol. 2017 Jun;47(6):946-953. doi: 10.1002/eji.201646837. Eur J Immunol. 2017. PMID: 28475283 Free PMC article. Review.
-
CD4+ T cells in inflammatory diseases: pathogenic T-helper cells and the CD69-Myl9 system.Int Immunol. 2021 Nov 25;33(12):699-704. doi: 10.1093/intimm/dxab053. Int Immunol. 2021. PMID: 34427648 Review.
Cited by
-
A Phase 2 Study to Assess the Immunomodulatory Capacity of a Lecithin-based Delivery System of Curcumin in Endometrial Cancer.Front Nutr. 2019 Jan 11;5:138. doi: 10.3389/fnut.2018.00138. eCollection 2018. Front Nutr. 2019. PMID: 30687714 Free PMC article.
-
CD69 is the crucial regulator of intestinal inflammation: a new target molecule for IBD treatment?J Immunol Res. 2015;2015:497056. doi: 10.1155/2015/497056. Epub 2015 Feb 22. J Immunol Res. 2015. PMID: 25759842 Free PMC article. Review.
-
Promising role of curcumin against viral diseases emphasizing COVID-19 management: A review on the mechanistic insights with reference to host-pathogen interaction and immunomodulation.J Funct Foods. 2021 Jul;82:104503. doi: 10.1016/j.jff.2021.104503. Epub 2021 Apr 20. J Funct Foods. 2021. PMID: 33897833 Free PMC article. Review.
-
Local administration of curcumin-loaded nanoparticles effectively inhibits inflammation and bone resorption associated with experimental periodontal disease.Sci Rep. 2018 Apr 27;8(1):6652. doi: 10.1038/s41598-018-24866-2. Sci Rep. 2018. PMID: 29703905 Free PMC article.
-
Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signaling.Cancer Immunol Immunother. 2014 Sep;63(9):889-900. doi: 10.1007/s00262-014-1564-5. Epub 2014 Jun 4. Cancer Immunol Immunother. 2014. PMID: 24893859 Free PMC article.
References
-
- Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, et al. (2005) Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol 174: 8116–8124. - PubMed
-
- Jeong YI, Kim SW, Jung ID, Lee JS, Chang JH, et al. (2009) Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the Janus-activated kinase-protein kinase Cdelta-STAT1 signaling pathway in interferon-gamma-stimulated murine dendritic cells. J Biol Chem 284: 3700–3708. - PubMed
-
- Cong Y, Wang L, Konrad A, Schoeb T, Elson CO (2009) Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur J Immunol 39: 3134–3146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

