Candidate gene study of TRAIL and TRAIL receptors: association with response to interferon beta therapy in multiple sclerosis patients
- PMID: 23658636
- PMCID: PMC3639207
- DOI: 10.1371/journal.pone.0062540
Candidate gene study of TRAIL and TRAIL receptors: association with response to interferon beta therapy in multiple sclerosis patients
Abstract
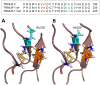
TRAIL and TRAIL Receptor genes have been implicated in Multiple Sclerosis pathology as well as in the response to IFN beta therapy. The objective of our study was to evaluate the association of these genes in relation to the age at disease onset (AAO) and to the clinical response upon IFN beta treatment in Spanish MS patients. We carried out a candidate gene study of TRAIL, TRAILR-1, TRAILR-2, TRAILR-3 and TRAILR-4 genes. A total of 54 SNPs were analysed in 509 MS patients under IFN beta treatment, and an additional cohort of 226 MS patients was used to validate the results. Associations of rs1047275 in TRAILR-2 and rs7011559 in TRAILR-4 genes with AAO under an additive model did not withstand Bonferroni correction. In contrast, patients with the TRAILR-1 rs20576-CC genotype showed a better clinical response to IFN beta therapy compared with patients carrying the A-allele (recessive model: p = 8.88×10(-4), pc = 0.048, OR = 0.30). This SNP resulted in a non synonymous substitution of Glutamic acid to Alanine in position 228 (E228A), a change previously associated with susceptibility to different cancer types and risk of metastases, suggesting a lack of functionality of TRAILR-1. In order to unravel how this amino acid change in TRAILR-1 would affect to death signal, we performed a molecular modelling with both alleles. Neither TRAIL binding sites in the receptor nor the expression levels of TRAILR-1 in peripheral blood mononuclear cell subsets (monocytes, CD4+ and CD8+ T cells) were modified, suggesting that this SNP may be altering the death signal by some other mechanism. These findings show a role for TRAILR-1 gene variations in the clinical outcome of IFN beta therapy that might have relevance as a biomarker to predict the response to IFN beta in MS.
Conflict of interest statement
Figures


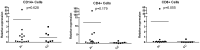
Similar articles
-
TRAIL and TRAIL receptors splice variants during long-term interferon β treatment of patients with multiple sclerosis: evaluation as biomarkers for therapeutic response.J Neurol Neurosurg Psychiatry. 2016 Feb;87(2):130-7. doi: 10.1136/jnnp-2014-309932. Epub 2015 Mar 3. J Neurol Neurosurg Psychiatry. 2016. PMID: 25736057 Free PMC article.
-
TRAIL/TRAIL receptor system and susceptibility to multiple sclerosis.PLoS One. 2011;6(7):e21766. doi: 10.1371/journal.pone.0021766. Epub 2011 Jul 21. PLoS One. 2011. PMID: 21814551 Free PMC article.
-
Hepatocyte expression of TRAIL pathway regulators correlates with histopathological and clinical parameters in chronic HCV infection.Pathol Res Pract. 2014 Feb;210(2):83-91. doi: 10.1016/j.prp.2013.10.005. Epub 2013 Nov 7. Pathol Res Pract. 2014. PMID: 24268735
-
[Progress on targeting TRAIL's receptor as antitumor strategy].Yao Xue Xue Bao. 2009 Dec;44(12):1336-42. Yao Xue Xue Bao. 2009. PMID: 21351465 Review. Chinese.
-
TRAIL receptor signalling and modulation: Are we on the right TRAIL?Cancer Treat Rev. 2009 May;35(3):280-8. doi: 10.1016/j.ctrv.2008.11.006. Epub 2008 Dec 30. Cancer Treat Rev. 2009. PMID: 19117685 Review.
Cited by
-
From periphery to center stage: 50 years of advancements in innate immunity.Cell. 2024 Apr 25;187(9):2030-2051. doi: 10.1016/j.cell.2024.03.036. Cell. 2024. PMID: 38670064 Free PMC article. Review.
-
Pharmacogenetic Biomarkers to Predict Treatment Response in Multiple Sclerosis: Current and Future Perspectives.Mult Scler Int. 2017;2017:6198530. doi: 10.1155/2017/6198530. Epub 2017 Jul 19. Mult Scler Int. 2017. PMID: 28804651 Free PMC article. Review.
-
Pharmacogenetic Predictors of Response to Interferon Beta Therapy in Multiple Sclerosis.Mol Neurobiol. 2021 Sep;58(9):4716-4726. doi: 10.1007/s12035-021-02454-2. Epub 2021 Jun 24. Mol Neurobiol. 2021. PMID: 34169444
-
Pharmacogenomics of Multiple Sclerosis: A Systematic Review.Front Neurol. 2019 Feb 26;10:134. doi: 10.3389/fneur.2019.00134. eCollection 2019. Front Neurol. 2019. PMID: 30863357 Free PMC article. Review.
References
-
- McKeage K (2008) Interferon-beta-1b: in newly emerging multiple sclerosis. CNS Drugs 22: 787–792. - PubMed
-
- Doggrell SA (2010) Good results for early treatment of clinically isolated syndrome prior to multiple sclerosis with interferon beta-1b and glatiramer group. Expert Opin Pharmacother 11: 1225–1230. - PubMed
-
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352: 1498–1504. - PubMed
-
- Fernandez O, Arbizu T, Izquierdo G, Martinez-Yelamos A, Gata JM, et al. (2003) Clinical benefits of interferon beta-1a in relapsing-remitting MS: a phase IV study. Acta Neurol Scand 107: 7–11. - PubMed
-
- Rio J, Nos C, Tintore M, Tellez N, Galan I, et al. (2006) Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol 59: 344–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

