A lympho-follicular microenvironment is required for pathological prion protein deposition in chronically inflamed tissues from scrapie-affected sheep
- PMID: 23658779
- PMCID: PMC3643908
- DOI: 10.1371/journal.pone.0062830
A lympho-follicular microenvironment is required for pathological prion protein deposition in chronically inflamed tissues from scrapie-affected sheep
Abstract
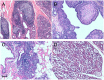
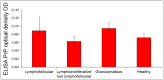
In sheep scrapie, pathological prion protein (PrP(Sc)) deposition occurs in the lymphoreticular and central nervous systems. We investigated PrP(Sc) distribution in scrapie-affected sheep showing simultaneous evidence of chronic lymphofollicular, lymphoproliferative/non-lymphofollicular, and/or granulomatous inflammations in their mammary gland, lung, and ileum. To do this, PrP(Sc) detection was carried out via immunohistochemistry and Western Blotting techniques, as well as through inflammatory cell immunophenotyping. Expression studies of gene coding for biological factors modulating the host's inflammatory response were also carried out. We demonstrated that ectopic PrP(Sc) deposition occurs exclusively in the context of lymphofollicular inflammatory sites, inside newly formed and well-organized lymphoid follicles harboring follicular dendritic cells. On the contrary, no PrP(Sc) deposition was detected in granulomas, even when they were closely located to newly formed lymphoid follicles. A significantly more consistent expression of lymphotoxin α and β mRNA was detected in lymphofollicular inflammation compared to the other two types, with lymphotoxin α and β signaling new lymphoid follicles' formation and, likely, the occurrence of ectopic PrP(Sc) deposition inside them. Our findings suggest that, in sheep co-affected by scrapie and chronic inflammatory conditions, only newly formed lymphoid follicles provide a suitable micro-environment that supports the scrapie agent's replication in inflammatory sites, with an increased risk of prion shedding through body secretions/excretions.
Conflict of interest statement
Figures






References
-
- Andréoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, et al. (2000) Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol 81: 3115–3126. - PubMed
-
- Andréoletti O, Simon S, Lacroux C, Morel N, Tabouret G, et al. (2004) PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat Med 10: 591–593. - PubMed
-
- Andréoletti O, Lacroux C, Chabert A, Monnereau L, Tabouret G, et al. (2002) PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol 83: 2607–2616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

