Development of M1 mAChR allosteric and bitopic ligands: prospective therapeutics for the treatment of cognitive deficits
- PMID: 23659787
- PMCID: PMC3715844
- DOI: 10.1021/cn400086m
Development of M1 mAChR allosteric and bitopic ligands: prospective therapeutics for the treatment of cognitive deficits
Abstract
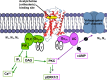
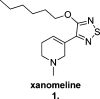
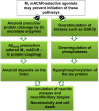
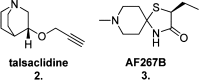
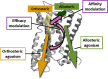
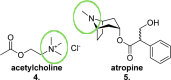
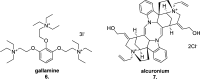
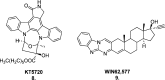
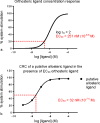
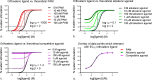
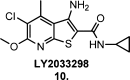
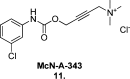
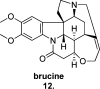
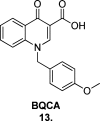
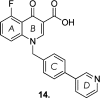
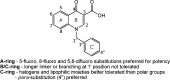
Since the cholinergic hypothesis of memory dysfunction was first reported, extensive research efforts have focused on elucidating the mechanisms by which this intricate system contributes to the regulation of processes such as learning, memory, and higher executive function. Several cholinergic therapeutic targets for the treatment of cognitive deficits, psychotic symptoms, and the underlying pathophysiology of neurodegenerative disorders, such as Alzheimer's disease and schizophrenia, have since emerged. Clinically approved drugs now exist for some of these targets; however, they all may be considered suboptimal therapeutics in that they produce undesirable off-target activity leading to side effects, fail to address the wide variety of symptoms and underlying pathophysiology that characterize these disorders, and/or afford little to no therapeutic effect in subsets of patient populations. A promising target for which there are presently no approved therapies is the M1 muscarinic acetylcholine receptor (M1 mAChR). Despite avid investigation, development of agents that selectively activate this receptor via the orthosteric site has been hampered by the high sequence homology of the binding site between the five muscarinic receptor subtypes and the wide distribution of this receptor family in both the central nervous system (CNS) and the periphery. Hence, a plethora of ligands targeting less structurally conserved allosteric sites of the M1 mAChR have been investigated. This Review aims to explain the rationale behind allosterically targeting the M1 mAChR, comprehensively summarize and critically evaluate the M1 mAChR allosteric ligand literature to date, highlight the challenges inherent in allosteric ligand investigation that are impeding their clinical advancement, and discuss potential methods for resolving these issues.
Figures

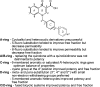
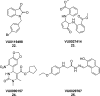
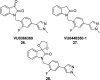
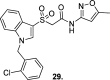
References
-
- van Koppen C. J.; Kaiser B. (2003) Regulation of muscarinic acetylcholine receptor signaling. Pharmacol. Ther. 98, 197–220. - PubMed
-
- Canals M.; Sexton P. M.; Christopoulos A. (2011) Allostery in GPCRs: “MWC” revisited. Trends Biochem. Sci. 36, 663–672. - PubMed
-
- Caulfield M. P. (1993) Muscarinic Receptors-Characterization, Coupling and Function. Pharmacol. Ther. 58, 319–379. - PubMed
-
- Fisher A. (2008) M1 Muscarinic Agonists Target Major Hallmarks of Alzheimer’s Disease - The Pivotal Role of Brain M1 Receptors. Neurodegener. Dis. 5, 237–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

