Neuronal morphology in MeCP2 mouse models is intrinsically variable and depends on age, cell type, and Mecp2 mutation
- PMID: 23659895
- PMCID: PMC3748238
- DOI: 10.1016/j.nbd.2013.04.020
Neuronal morphology in MeCP2 mouse models is intrinsically variable and depends on age, cell type, and Mecp2 mutation
Abstract
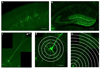
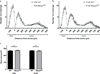
Rett Syndrome (RTT), a progressive neurological disorder characterized by developmental regression and loss of motor and language skills, is caused by mutations in the X-linked gene encoding methyl-CpG binding protein 2 (MECP2). Neurostructural phenotypes including decreased neuronal size, dendritic complexity, and spine density have been reported in postmortem RTT brain tissue and in Mecp2 animal models. How these changes in neuronal morphology are related to RTT-like phenotype and MeCP2 function, and the extent to which restoration of neuronal morphology can be used as a cellular readout in therapeutic studies, however, remain unclear. Here, we systematically examined neuronal morphology in vivo across three Mecp2 mouse models representing Mecp2 loss-of-function, partial loss-of-function, and gain-of-function mutations, at developmental time points corresponding to early- and late-symptomatic RTT-like behavioral phenotypes. We found that in Mecp2 loss-of-function mouse models, dendritic complexity is reduced in a mild, age-dependent, and brain region-specific manner, whereas soma size is reduced consistently throughout development. Neither phenotype, however, is altered in Mecp2 gain-of-function mice. Our results suggest that, in the cell types we examined, the use of dendritic morphology as a cellular readout of RTT phenotype and therapeutic efficacy should be cautioned, as it is intrinsically variable. In contrast, soma size may be a robust and reliable marker for evaluation of MeCP2 function in Mecp2 loss-of-function studies.
Keywords: Dendritic branching; MECP2; Mouse model; Neuronal morphology; Rett syndrome; Soma size.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



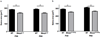
Similar articles
-
Abnormalities of cell packing density and dendritic complexity in the MeCP2 A140V mouse model of Rett syndrome/X-linked mental retardation.BMC Neurosci. 2010 Feb 17;11:19. doi: 10.1186/1471-2202-11-19. BMC Neurosci. 2010. PMID: 20163734 Free PMC article.
-
MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice.PLoS One. 2012;7(3):e31896. doi: 10.1371/journal.pone.0031896. Epub 2012 Mar 7. PLoS One. 2012. PMID: 22412847 Free PMC article.
-
Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks.J Comp Neurol. 2009 May 20;514(3):240-58. doi: 10.1002/cne.22009. J Comp Neurol. 2009. PMID: 19296534
-
Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review.
-
Rett syndrome: from bed to bench.Pediatr Neonatol. 2011 Dec;52(6):309-16. doi: 10.1016/j.pedneo.2011.08.002. Epub 2011 Nov 6. Pediatr Neonatol. 2011. PMID: 22192257 Review.
Cited by
-
Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development.Brain Res. 2020 Feb 15;1729:146644. doi: 10.1016/j.brainres.2019.146644. Epub 2020 Jan 2. Brain Res. 2020. PMID: 31904347 Free PMC article. Review.
-
Apoptotic Activity of MeCP2 Is Enhanced by C-Terminal Truncating Mutations.PLoS One. 2016 Jul 21;11(7):e0159632. doi: 10.1371/journal.pone.0159632. eCollection 2016. PLoS One. 2016. PMID: 27442528 Free PMC article.
-
Aberrant astrocyte protein secretion contributes to altered neuronal development in multiple models of neurodevelopmental disorders.Nat Neurosci. 2022 Sep;25(9):1163-1178. doi: 10.1038/s41593-022-01150-1. Epub 2022 Aug 30. Nat Neurosci. 2022. PMID: 36042312 Free PMC article.
-
Reduced neuronal size and mTOR pathway activity in the Mecp2 A140V Rett syndrome mouse model.F1000Res. 2016 Sep 8;5:2269. doi: 10.12688/f1000research.8156.1. eCollection 2016. F1000Res. 2016. PMID: 27781091 Free PMC article.
-
Hyperexcitability and brain morphological differences in mice lacking the cystine/glutamate antiporter, system xc.J Neurosci Res. 2021 Dec;99(12):3339-3353. doi: 10.1002/jnr.24971. Epub 2021 Nov 8. J Neurosci Res. 2021. PMID: 34747522 Free PMC article.
References
-
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. - PubMed
-
- Armstrong D, Dunn JK, Antalffy B, Trivedi R. Selective dendritic alterations in the cortex of Rett syndrome. J. Neuropathol. Exp. Neurol. 1995;54:195–201. - PubMed
-
- Armstrong DD. Neuropathology of Rett syndrome. Journal of Child Neurology. 2005;20:747–753. - PubMed
-
- Bauman ML, Kemper TL, Arin DM. Pervasive neuroanatomic abnormalities of the brain in three cases of Rett's syndrome. Neurology. 1995;45:1581–1586. - PubMed
-
- Belichenko NP, Belichenko PV, Mobley WC. Evidence for both neuronal cell autonomous and nonautonomous effects of methyl-CpG-binding protein 2 in the cerebral cortex of female mice with Mecp2 mutation. Neurobiol. Dis. 2009a;34:71–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

